Wavelight® Topography-Guided CONTOURA® Vision LASIK
Wavelight CONTOURA® Vision is a topography guided (TG) LASIK treatment that takes into account the shape of the anterior corneal surface and uses corneal height data from the corneal topography measures that are taken prior to surgery. These treatment profiles help manage higher order aberrations (including spherical aberration) of the cornea by compensating for cosine effect and by normalizing or ‘smoothing’ the overall surface of the cornea to improve its regularity. When these changes are made to smooth out the elevations on the cornea, it changes the overall refractive correction needed and may not match the original manifest refraction. This means that tweaks in the spherical and/or astigmatism components of the manifest refraction inputs will likely need to be changed when determining the final treatment calculation for CONTOURA® Vision. These changes can be done manually by the surgeon more or less guessing what the required change should be or, instead, the surgical planning can be simplified by using special software called Phorcides^. This software calculates the exact treatment profile required without manual input from the surgeon and takes the guesswork out of TG CONTOURA® Vision LASIK treatment planning.1,2
Phorcides^ Analytic Engine Software
The Phorcides^ software objectively determines the required treatment profile. It takes into account not only the anterior corneal astigmatism and the anterior topography irregularities that cause higher order aberrations but also posterior corneal astigmatism and lenticular astigmatism.2
Phorcides^ uses geographic imaging software to analyze the anterior topographic profile of an individual’s cornea. It takes into account anterior topographic data from the Placido Disc-based Topolyzer® Vario, along with anterior and posterior corneal astigmatism data from a Scheimpflug topographer. The Phorcides^ software measures the radius of each raised topographical feature, or talus, and uses all this information to determine the true anterior corneal astigmatism. The software compiles a series of refractive vectors: one vector for each raised topographic feature on the corneal surface, one for the anterior corneal astigmatism, one for the posterior corneal astigmatism, and one for any internal lenticular astigmatism. Finally, it uses a series of advanced computer algorithms to determine an optimized sphere and cylinder magnitude and orientation for the treatment.2
This more objective approach allows the treatment planning to be more efficient and the Phorcides^ software has shown improved vision outcomes compared to manual treatment planning, particularly at the 20/15 and better level.2,3
Clinical Study Assessing Patient Reported Outcomes after Wavelight® Topography-Guided CONTOURA® Vision LASIK using Phorcides^
Patient-reported outcomes are critical to evaluating post-LASIK results. In this prospective, observational, investigator-initiated trial, subjects underwent bilateral TG LASIK with CONTOURA® Vision, using the WaveLight® FS200 femtosecond laser and EX500 excimer laser and using Phorcides^ for surgical planning. Outcomes were measured at 26 weeks (6.5 months) post-operatively. We assessed subjective outcomes using a modified PROWL questionnaire, which is validated for assessment of patient satisfaction after LASIK. We also analyzed visual acuity and higher-order aberrations. In total, 46 subjects completed the study.4


At 26 weeks post-operatively, the modified PROWL survey showed significant improvement on the Global Vision Satisfaction Index where a score of one represented “least satisfied” and a score of five represented “most satisfied”. The average uncorrected post-operative score of five was a significant improvement over the corrected baseline average of 4.1 on the five-point scale. In addition, 100% of subjects reported the maximum score of five for overall satisfaction with their vision. Analysis of individual questionnaire items also showed significant improvements versus baseline. Subjects showed improvements with respect to vision while driving at night, symptoms of glare, halos, and starbursts, symptoms of dry eye, and improvement on the global symptom assessment.4
Post operative uncorrected distance visual acuity was also excellent with more subjects reaching 20/16 or better compared to preoperative best corrected vision. Binocular uncorrected distance acuity was 20/10 or better for 15% of subjects, 20/12.5 or better for 87% of subjects and 20/16 or better for 100% of subjects. Additionally, eighty-three percent of subjects gained at least one line of Snellen binocular best-corrected distance visual acuity, while the rest had no change.4
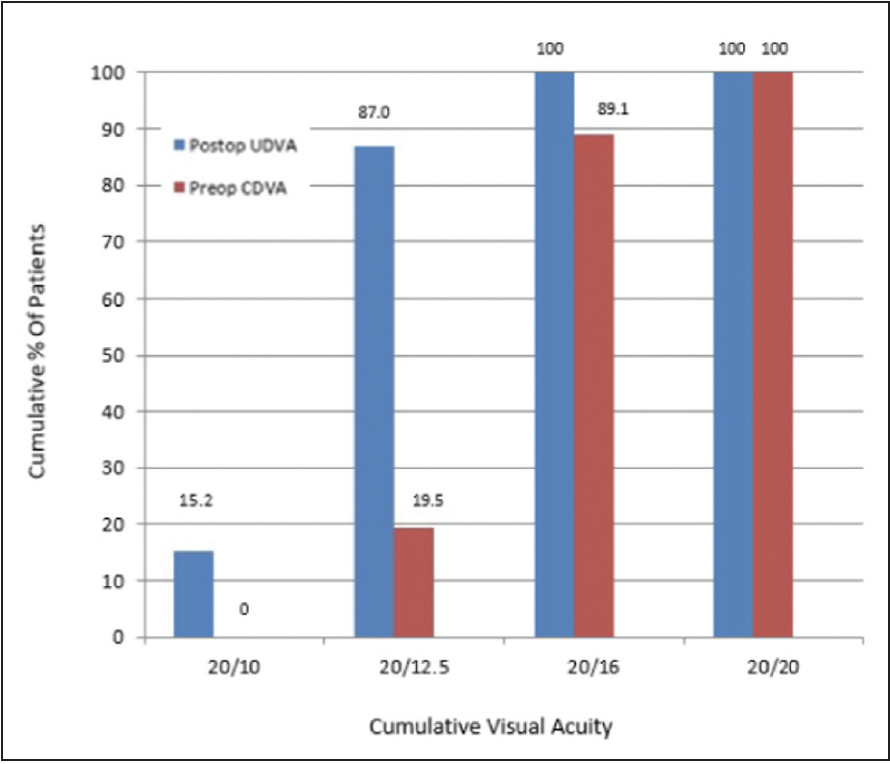
Figure 1. Binocular Visual Acuity @ 6.5 months; n=46 subjects
CDVA, best-corrected distance visual acuity; LASIK, laser-assisted in situ keratomileusis;
UDVA, uncorrected distance visual acuity.
This study also looked at the objective scatter index and total corneal higher-order aberrations at a four-millimeter optic zone. The results showed that there was no significant change from baseline to 6.5 months post-operatively.4
Conclusion
CONTOURA® Vision, and specifically the use of the Phorcides^ software, provides excellent visual outcomes particularly at the 20/15 or better level.2-5 The Phorcides^ software helps surgeons avoid the subjective nature of CONTOURA® Vision that can require determining the right balance of cylinder magnitude and axis, and it provides a more objective, simplified approach that makes it more appealing to surgeons. This study, assessing patient reported outcomes, shows that CONTOURA® Vision provides significant improvement in patient satisfaction after treatment.4 It was also key in demonstrating that CONTOURA® Vision TG LASIK with Phorcides^, provides the benefit of improving visual quality beyond what can be measured objectively with visual acuity. The use of Phorcides^ allows for easy and reliable planning for CONTOURA® Vision and shows exceptional satisfaction and visual outcomes that surgeons can be confident in.4
WAVELIGHT® EXCIMER LASER SYSTEMS IMPORTANT PRODUCT INFORMATION
This information pertains to all WaveLight® Excimer Laser Systems, including the WaveLight® ALLEGRETTO WAVE®, the ALLEGRETTO WAVE® Eye-Q and the WaveLight® EX500. Caution: Federal (U.S.) law restricts the WaveLight® Excimer Laser Systems to sale by or on the order of a physician. Only practitioners who are experienced in the medical mangement and surgical treatment of the cornea, who have been trained in laser refractive surgery (including laser calibration and operation) should use a WaveLight® Excimer Laser System. Indications: FDA has approved the WaveLight® Excimer Laser systems for use in laser-assisted in situ keratomileusis (LASIK) treatments for: the reduction or elimination of myopia of up to - 12.00 D and up to 6.00 D of astigmatism at the spectacle plane; the reduction or elimination of hyperopia up to + 6.00 D with and without astigmatic refractive errors up to 5.00 D at the spectacle plane, with a maximum manifest refraction spherical equivalent of + 6.00 D; • the reduction or elimination of naturally occurring mixed astigmatism of up to 6.00 D at the spectacle plane; and the wavefront-guided reduction or elimination of myopia of up to -7.00 D and up to 3.00 D of astigmatism at the spectacle plane. In addition, FDA has approved the WaveLight® ALLEGRETTO WAVE® Eye-Q Excimer Laser System, when used with the WaveLight® ALLEGRO Topolyzer® and topography-guided treatment planning software for topography-guided LASIK treatments for the reduction or elimination of up to -9.00 D of myopia, or for the reduction or elimination of myopia with astigmatism, with up to -8.00 D of myopia and up to 3.00 D of astigmatism. The WaveLight® Excimer Laser Systems are only indicated for use in patients who are 18 years of age or older (21 years of age or older for mixed astigmatism) with documentation of a stable manifest refraction defined as ≤ 0.50 D of preoperative spherical equivalent shift over one year prior to surgery, exclusive of changes due to unmasking latent hyperopia. Contraindications: The WaveLight® Excimer Laser Systems are contraindicated for use with patients who: are pregnant or nursing; have a diagnosed collagen vascular, autoimmune or immunodeficiency disease; have been diagnosed keratoconus or if there are any clinical pictures suggestive of keratoconus; are taking isotretinoin (Accutane*) and/or amiodarone hydrochloride (Cordarone*); have severe dry eye; have corneas too thin for LASIK; have recurrent corneal erosion; have advanced glaucoma; or have uncontrolled diabetes. Warnings: The WaveLight® Excimer Laser Systems are not recommended for use with patients who have: systemic diseases likely to affect wound healing, such as connective tissue disease, insulin dependent diabetes, severe atopic disease or an immunocompromised status; a history of Herpes simplex or Herpes zoster keratitis; significant dry eye that is unresponsive to treatment; severe allergies; a history of glaucoma; an unreliable preoperative wavefront examination that precludes wavefront-guided treatment; or a poor quality preoperative topography map that precludes topography-guided LASIK treatment. The wavefront-guided LASIK procedure requires accurate and reliable data from the wavefront examination. Every step of every wavefront measurement that may be used as the basis for a wavefront-guided LASIK procedure must be validated by the user. Inaccurate or unreliable data from the wavefront examination will lead to an inaccurate treatment. Topography-guided LASIK requires preoperative topography maps of sufficient quality to use for planning a topography-guided LASIK treatment. Poor quality topography maps may affect the accuracy of the topography-guided LASIK treatment and may result in poor vision after topography-guided LASIK. Precautions: The safety and effectiveness of the WaveLight® Excimer Laser Systems have not been established for patients with: progressive myopia, hyperopia, astigmatism and/or mixed astigmatism, ocular disease, previous corneal or intraocular surgery, or trauma in the ablation zone; corneal abnormalities including, but not limited to, scars, irregular astigmatism and corneal warpage; residual corneal thickness after ablation of less than 250 microns due to the increased risk for corneal ectasia; pupil size below 7.0 mm after mydriatics where applied for wavefront-guided ablation planning; history of glaucoma or ocular hypertension of > 23 mmHg; taking the medications sumatriptan succinate (Imitrex*); corneal, lens and/or vitreous opacities including, but not limited to cataract; iris problems including, but not limited to, coloboma and previous iris surgery compormising proper eye tracking; or taking medications likely to affect wound healing including (but not limited to) antimetabolites. In addition, safety and effectiveness of the WaveLight® Excimer Laser Systems have not been established for: treatments with an optical zone < 6.0 mm or > 6.5 mm in diameter, or an ablation zone > 9.0 mm in diameter; or wavefront-guided treatment targets different from emmetropia (plano) in which the wavefront calculated defocus (spherical term) has been adjusted; In the WaveLight® Excimer Laser System clinical studies, there were few subjects with cylinder amounts > 4 D and ≤ 6 D N. ot all complications, adverse events, and levels of effectiveness may have been determined for this population. Pupil sizes should be evaluated under mesopic illumination conditions. Effects of treatment on vision under poor illumination cannot be predicted prior to surgery. Adverse Events and Complications: Myopia: In the myopia clinical study, 0.2% (2/876) of the eyes had a lost, misplaced, or misaligned flap reported at the 1 month examination. The following complications were reported 6 months after LASIK: 0.9% (7/818) had ghosting or double images in the operative eye; 0.1% (1/818) of the eyes had a corneal epithelial defect. Hyperopia: In the hyperopia clinical study, 0.4% (1/276) of the eyes had a retinal detachment or retinal vascular accident reported at the 3 month examination. The following complications were reported 6 months after LASIK: 0.8% (2/262) of the eyes had a corneal epithelial defect and 0.8% (2/262) had any epithelium in the interface. Mixed Astigmatism: In the mixed astigmatism clinical study, two adverse events were reported. The first event involved a patient who postoperatively was subject to blunt trauma to the treatment eye 6 days after surgery. The patient was found to have an intact globe with no rupture, inflammation or any dislodgement of the flap. UCVA was decreased due to this event. The second event involved the treatment of an incorrect axis of astigmatism. The axis was treated at 60 degrees instead of 160 degrees. The following complications were reported 6 months after LASIK: 1.8% (2/111) of the eyes had ghosting or double images in the operative eye. Wavefront-Guided Myopia: The wavefront-guided myopia clinical study included 374 eyes treated; 188 with wavefront-guided LASIK (Study Cohort) and 186 with Wavefront Optimized® LASIK (Control Cohort). No adverse events occurred during the postoperative period of the wavefront-guided LASIK procedures. In the Control Cohort, one subject undergoing traditional LASIK had the axis of astigmatism programmed as 115 degrees instead of the actual 155 degree axis. This led to cylinder in the left eye. The following complications were reported 6 months after wavefront-guided LASIK in the Study Cohort: 1.2% (2/166) of the eyes had a corneal epithelial defect; 1.2% (2/166) had foreign body sensation; and 0.6% (1/166) had pain. No complications were reported in the Control Cohort. Topography-Guided Myopia: There were six adverse events reported in the topography-guided myopia study. Four of the eyes experienced transient or temporary decreases in vision prior to the final 12 month follow-up visit, all of which were resolved by the final follow-up visit. One subject suffered from decreased vision in the treated eye, following blunt force trauma 4 days after surgery. One subject experienced retinal detachment, which was concluded to be unrelated to the surgical procedure. Clinical Data: Myopia: The myopia clinical study included 901 eyes treated, of which 813 of 866 eligible eyes were followed for 12 months. Accountability at 3 months was 93.8%, at 6 months was 91.9%, and at 12 months was 93.9%. Of the 782 eyes that were eligible for the uncorrected visual acuity (UCVA) analysis of effectiveness at the 6-month stability time point, 98.3% were corrected to 20/40 or better, and 87.7% were corrected to 20/20 or better. Subjects who responded to a patient satisfaction questionnaire before and after LASIK reported the following visual symptoms at a “moderate” or “severe” level at least 1% higher at 3 months post-treatment than at baseline: visual fluctuations (28.6% vs. 12.8% at baseline). Long term risks of LASIK for myopia with and without astigmatism have not been studied beyond 12 months. Hyperopia: The hyperopia clinical study included 290 eyes treated, of which 100 of 290 eligible eyes were followed for 12 months. Accountability at 3 months was 95.2%, at 6 months was 93.9%, and at 12 months was 69.9%. Of the 212 eyes that were eligible for the UCVA analysis of effectiveness at the 6-month stability time point, 95.3% were corrected to 20/40 or better, and 69.4% were corrected to 20/20 or better. Subjects who responded to a patient satisfaction questionnaire before and after LASIK reported the following visual symptoms as “much worse” at 6 months post-treatment: halos (6.4%); visual fluctuations (6.1%); light sensitivity (4.9%); night driving glare (4.2%); and glare from bright lights (3.0%). Long term risks of LASIK for hyperopia with and without astigmatism have not been studied beyond 12 months. Mixed Astigmatism: The mixed astigmatism clinical study included 162 eyes treated, of which 111 were eligible to be followed for 6 months. Accountability at 1 month was 99.4%, at 3 months was 96.0%, and at 6 months was 100.0%. Of the 142 eyes that were eligible for the UCVA analysis of effectiveness at the 6-month stability time point, 97.3% achieved acuity of 20/40 or better, and 69.4% achieved acuity of 20/20 or better. Subjects who responded to a patient satisfaction questionnaire before and after LASIK reported the following visual symptoms at a “moderate” or “severe” level at least 1% higher at 3 months post-treatment than at baseline: sensitivity to light (52.9% vs. 43.3% at baseline); visual fluctuations (43.0% vs. 32.1% at baseline); and halos (42.3% vs. 37.0% at baseline). Long term risks of LASIK for mixed astigmatism have not been studied beyond 6 months. Wavefront-Guided Myopia: The wavefront-guided myopia clinical study included 374 eyes treated; 188 with wavefront-guided LASIK (Study Cohort) and 186 with Wavefront Optimized® LASIK (Control Cohort). 166 of the Study Cohort and 166 of the Control Cohort were eligible to be followed at 6 months. In the Study Cohort, accountability at 1 month was 96.8%, at 3 months was 96.8%, and at 6 months was 93.3%. In the Control Cohort, accountability at 1 month was 94.6%, at 3 months was 94.6%, and at 6 months was 92.2%. Of the 166 eyes in the Study Cohort that were eligible for the UCVA analysis of effectiveness at the 6-month stability time point, 99.4% were corrected to 20/40 or better, and 93.4% were corrected to 20/20 or better. Of the 166 eyes in the Control Cohort eligible for the UCVA analysis of effectiveness at the 6-month stability time point, 99.4% were corrected to 20/40 or better, and 92.8% were corrected to 20/20. In the Study Cohort, subjects who responded to a patient satisfaction questionnaire before and after LASIK reported the following visual symptoms at a “moderate” or “severe” level at least 1% higher at 3 months post-treatment than at baseline: light sensitivity (47.8% vs. 37.2% at baseline) and visual fluctuations (20.0% vs. 13.8% at baseline). In the Control Cohort, the following visual symptoms were reported at a “moderate” or “severe” level at least 1% higher at 3 months post-treatment than at baseline: halos (45.4% vs. 36.6% at baseline) and visual fluctuations (219% vs. 18.3% at baseline). Long term risks of wavefront-guided LASIK for myopia with and without astigmatism have not been studied beyond 6 months. Topography-Guided Myopia: The topography-guided myopia clinical study included 249 eyes treated, of which 230 eyes were followed for 12 months. Accountability at 3 months was 99.2%, at 6 months was 98.0%, and at 12 months was 92.4%. Of the 247 eyes that were eligible for the UCVA analysis at the 3-month stability time point, 99.2% were corrected to 20/40 or better, and 92.7% were corrected to 20/20 or better. Subjects who responded to a patient satisfaction questionnaire before and after LASIK reported the following visual symptoms as “marked” or “severe” at an incidence greater than 5% at 1 month after surgery: dryness (7% vs. 4% at baseline) and light sensitivity (7% vs. 5% at baseline). Visual symptoms continued to improve with time, and none of the visual symptoms were rated as being “marked” or “severe” with an incidence of at least 5% at 3 months or later after surgery. Long term risks of topography-guided LASIK for myopia with and without astigmatism have not been studied beyond 12 months. Information for Patients: Prior to undergoing LASIK surgery with a WaveLight® Excimer Laser System, prospective patients must receive a copy of the relevant Patient Information Booklet, and must be informed of the alternatives for correcting their vision, including (but not limited to) eyeglasses, contact lenses, photorefractive keratectomy, and other refractive surgeries. Attention: Please refer to a current WaveLight® Excimer Laser System Procedure Manual for a complete listing of the indications, complications, warnings, precautions, and side effects.
^ Trademark is the property of its respective owner
1. Stulting D, Durrie D, Potvin R, Linn D, Krueger R, Lobanoff M, Moshirfar M, Motwani M, Lindquist T, Stonecipher K. Topography-Guided Refractive Astigmatism Outcomes: Predictions Comparing Three Different Programming Methods. Clinical Ophthalmology. 2020;14:1091-1100.
2. Lobanoff M, Stonecipher K, Tooma T, Wexler S, Potvin R. Clinical Outcomes after Topography-Guided LASIK: Comparing Results Based on a New Topography Analysis Algorithm to Those Based on the Manifest Refraction. J Cataract Refract Surg. 2020;46(6):814-819.
3. Brunson P, Mann P, Mann P, Potvin R. Clinical Outcomes After Topography-Guided Refractive Surgery in Eyes with Myopia and Astigmatism – Comparing Results with New Planning Software to Those Obtained Using the Manifest Refraction. Clinical Ophthalmology. 2020:14 3975–3982.
4. Rush SW, Pickett CJ, Wilson BJ, Rush RB. Topography-Guided LASIK: A Prospective Study Evaluating Patient-Reported Outcomes. Clin Ophthalmol. 2023;17:2815-2824
5. Stulting RD, Lobanoff M, Mann P, Wexler S, Stonecipher K, Potvin R. Clinical and refractive outcomes after topography-guided refractive surgery planned using Phorcides surgery planning software. J Cataract Refract Surg 2022; 48:1010–1015.
© 2024 Alcon Inc. 1/24 US-WLC-2400002




