Moderator

Richard L. Lindstrom, MD
• Founder, Attending Surgeon, Minnesota Eye Consultants, Bloomington, Minnesota
• rllindstrom@mneye.com
• Financial disclosure: Consultant, Equity Interest (Omeros)
Panel

Patti Barkey, COE
• Practice Administrator, Chief Executive Officer, Certified Ophthalmic Executive, and Ophthalmic Coding Specialist, Bowden Eye & Associates, Jacksonville, Florida
• ASOA Board of Directors, Dry Eye University, Director
• pattibarkey@hotmail.com
• Financial disclosure: Consultant (Omeros)

Steven M. Silverstein, MD, FACS
• President, Silverstein Eye Centers, Kansas City, Missouri
• ssilverstein@silversteineyecenters.com
• Financial disclosure: Consultant (Omeros)

Denise M. Visco, MD, MBA
• Medical Director and Founder, Eyes of York Cataract & Laser Center, York, Pennsylvania
• dvisco@eyesofyork.com
• Financial disclosure: Consultant (Omeros)
• Omeros has provided financial support and medical writing support for studies by Dr. Visco.

Keith A. Walter, MD, FACS
• Professor of Ophthalmology, Wake Forest School of Medicine, Winston-Salem, North Carolina
• kwalter@wakehealth.edu
• Financial disclosure: Consultant (Omeros)
See Important Safety Information >>
The following discussion of OMIDRIA includes both data supported by clinical studies conducted as part of the FDA approval process for OMIDRIA and information based on real-world studies (Figure 1). The physicians in this group collectively have published and presented post-launch clinical studies (i.e., not included in current labeling) with prospective, retrospective, double-masked, open-label, cohort, case-controlled, single- and multi-center designs.
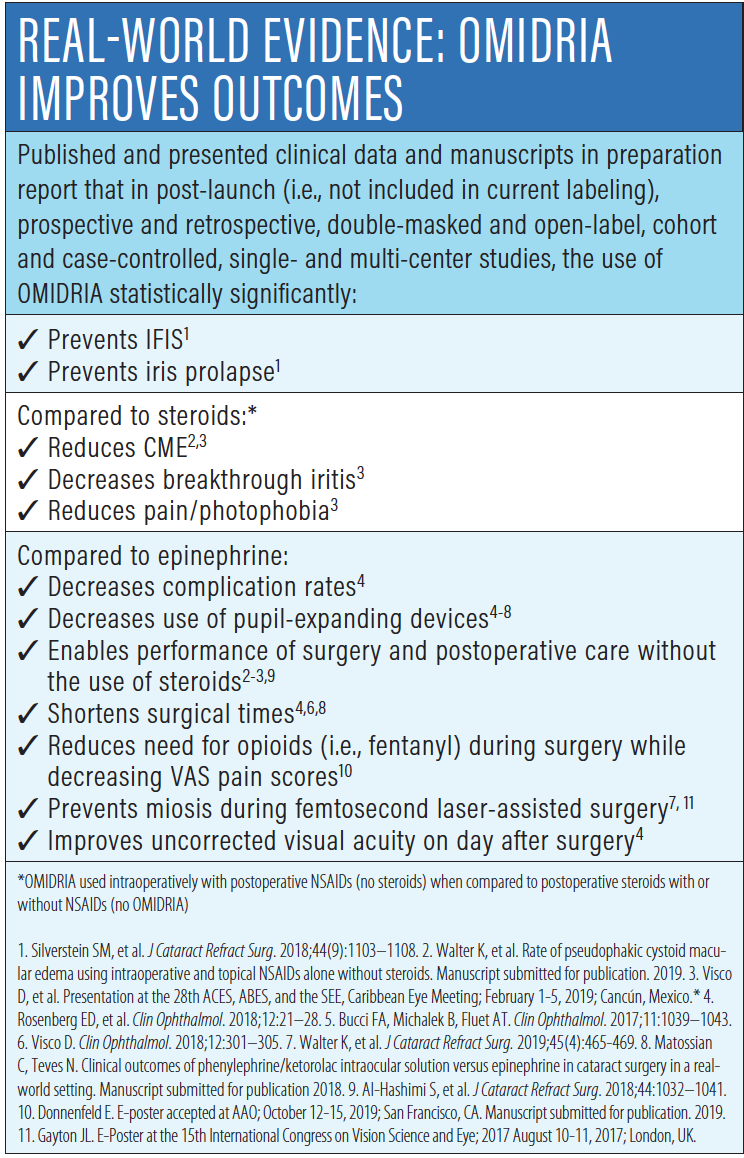
Figure 1. Real-world evidence shows that OMIDRIA delivers proven results.
Richard L. Lindstrom, MD: Surgical trauma from cataract surgery triggers an inflammatory cascade in which COX-1 and COX-2 enzymes release prostaglandin, resulting in inflammation.1,2This can lead to intraoperative miosis, postoperative pain, cystoid macular edema (CME), breakthrough iritis, and photophobia.3 Dealing with these problems can be costly, worsen outcomes, and prolong surgical time. Which one of these five problems would you say is the primary reason you use OMIDRIA?
Steven M. Silverstein, MD, FACS: OMIDRIA is very beneficial for all five of those findings, but from the surgeon’s perspective, the most significant is intraoperative miosis. Intraoperative miosis triggers the issues that lead to the other four problems. Anything we can do to control pupil dilation, maintain iris tone during surgery, and generate less prostaglandins will decrease other complications. It all begins with intraoperative miosis.
Keith A. Walter, MD, FACS: Yes, my number one reason for using OMIDRIA is prevention of intraoperative miosis because it’s an unpredictable problem. There are many cases when we don’t suspect it will happen, but it does, and it can make cases much more challenging.
Denise M. Visco, MD, MBA: I also agree that intraoperative miosis is the primary reason to use OMIDRIA, but the other problems you mentioned also bother me a great deal. When patients make repeated visits for CME or breakthrough iritis, that’s not particularly pleasant. OMIDRIA has changed my perspective on these complications. After 20 years in practice, I was very used to dealing with these problems. I’d accepted that intraoperative miosis happens, and we see patients with CME—we just need to deal with it. When I started using OMIDRIA, those complications diminished, and I began approaching them with a greater sense of control, rather than acceptance.
Dr. Lindstrom: I agree, and I think what causes me to reach for OMIDRIA is the desire to enhance the intraoperative experience by maintaining pupil dilation. Miosis during cataract surgery can result in increased surgical complications and surgical time, as well as worse patient outcomes due to poor visualization of the operative field.2,4,5 I think a lot of surgeons will say that pupil size is one of the most critical issues for successful cataract surgery. Risk of intraoperative miosis increases with a patient history of use of an alpha-blocker like tamsulosin, as well as other ocular comorbidities, such as uveitis, diabetes, previous ocular trauma, or pseudoexfoliation.6-11 As we mentioned, intraoperative miosis is also unpredictable, occurring in about 25% of patients with no risk factors.8 After surgery, patients with miosis report more pain and lower satisfaction.4,5
Can you describe how important it is for you to have a large pupil during cataract surgery?
Dr. Visco: Maintaining dilation is very important, not only to minimize complications, but also to keep the OR flowing smoothly because miosis affects surgery times. If we know a case will be complicated, we schedule more time, but when miosis is unexpected, we can suddenly be 20 or 30 minutes behind. If that happens several times in 1 day, it creates a problem for everyone in the surgery center.
Dr. Silverstein: Yes, it’s critical to have a large pupil. To have a poorly dilated pupil or a miotic pupil that’s decreasing in size during surgery dramatically increases the risk profile for trauma to the iris, capsular rupture, and vitreous prolapse.12 All of these complications trigger the inflammatory cycle with the release of more prostaglandins, which in turn leads to a higher incidence of macular edema. The smaller pupil also increases the potential for retained lens fragments and cortex.2
Dr. Walter: There are so many causes of an inadequate pupil during cataract surgery that the history, which is often unclear, is almost irrelevant to whether I choose OMIDRIA. I test patients by dilating them in the clinic to see if they get past 6 mm. If they do, I have greater confidence, but some patients who get to 7 mm or 8 mm end up being less than 6 mm dilated in the OR. It increases my anxiety and slows everything down, and sometimes I need to use mechanical devices. On the other hand, if I use OMIDRIA in these cases, then I don’t have to worry about that problem.
Dr. Lindstrom: We’ve talked about the surgeon’s perspective. How does an occurrence of miosis during surgery affect the patient’s experience?
Patti Barkey, COE: What comes to mind are patients having cataract surgery with ‘premium’ intraocular lenses and the femtosecond laser. If the pupil doesn’t dilate adequately, we have to tell the patient that the surgeon couldn’t use femtosecond technology. By using OMIDRIA for all our premium cataract patients, we are able to prevent miosis and perform the procedure as discussed with, and expected by, the patient.
Preventing Miosis
Dr. Lindstrom: It is certainly better to prevent miosis than to manage it, but this has proven to be a challenging goal. We can address miosis using mechanical methods, but we risk injury to the iris.2,6 Preoperative topical dilating agents can be helpful as well, but topical mydriatics can lose effectiveness as they’re washed out during the case.13 Compounded products carry some risks as well.14,15
OMIDRIA addresses miosis and postoperative pain through inhibition of inflammation-causing prostaglandins.16 It is added to the ophthalmic irrigating solution used during cataract surgery or intraocular lens replacement as indicated for maintaining pupil size.16 The proprietary combination of a mydriatic agent, phenylephrine, and ketorolac, an NSAID, in OMIDRIA offers complementary mechanisms of action: activating alpha1-adrenergic receptors and blocking inflammation by inhibiting prostaglandin release.16 Together, they work to maintain iris tone, prevent miosis, and reduce postoperative pain.16
Let’s say we have a patient with known intraoperative floppy iris syndrome (IFIS). Dr. Visco, how does OMIDRIA help that patient?
Dr. Visco: Using OMIDRIA has decreased problems with IFIS and allowed surgery to go more smoothly (Figure 2).17 With OMIDRIA, the iris actually becomes more rigid throughout the procedure, with less movement. There is less iris capture in the incisions or the phaco, and fewer lens fragments trapped beneath the iris.
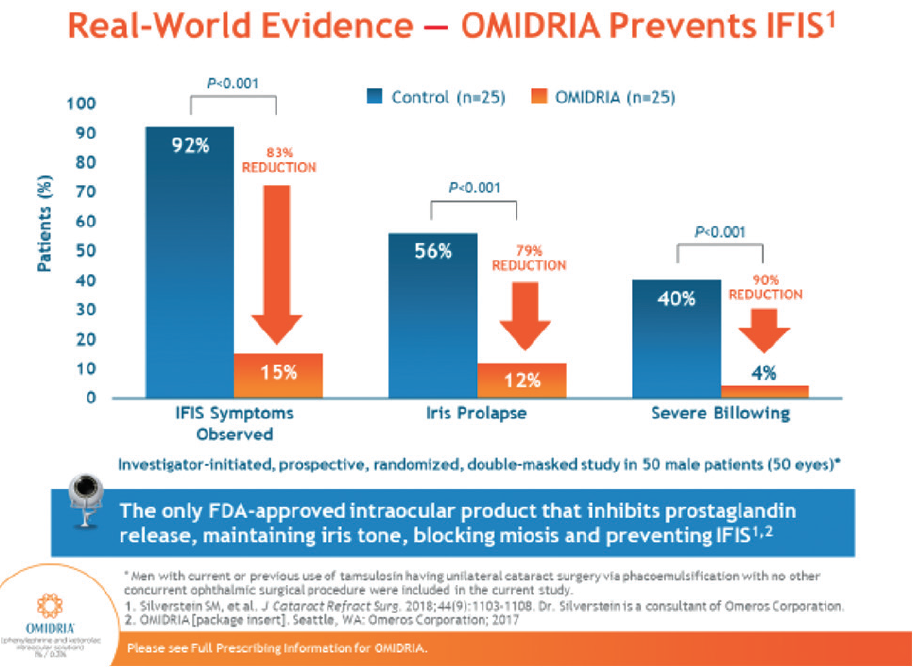
Figure 2. In a prospective, controlled, double-masked study of 50 male patients at risk for IFIS, 15% of patients using OMIDRIA had IFIS symptoms observed, compared to 92% of patients not using OMIDRIA.
Dr. Walter: I totally agree. Even in cases where I need to use a mechanical device in addition to OMIDRIA, such as a small, floppy pupil that doesn’t dilate, I find when I insert the ring that the tone of the iris is much stiffer and easier to manage. When the pupil isn’t too small, OMIDRIA achieves all the benefits we see in the peer-reviewed, published literature–better tone, preventing iris prolapse, and less billowing.17
Dr. Silverstein: The only way we know for certain that a patient has IFIS is if we’ve seen it in the fellow eye, but we know that the risk is higher in people taking tamsulosin or a similar medication. Plus diabetes, past history of iritis, age, infection, and trauma all affect whether an eye will dilate or stay dilated. In a recent study, we were able to demonstrate that pupils maintained better dilation throughout surgery with OMIDRIA.17 In my experience, using epinephrine is modestly helpful at best in helping maintain pupil dilation. Phenylephrine is a stronger agent. Phenylephrine and ketorolac both help maintain pupil dilation, and the combination is very effective.
Dr. Lindstrom: Compared to epinephrine, OMIDRIA reduces complication rates,2,18 decreases use of pupil-expanding devices,2,12,19-21 and cuts surgical times (Figure 3).2,19,20 What does that mean to a practice and an ASC in terms of efficiency and economics?
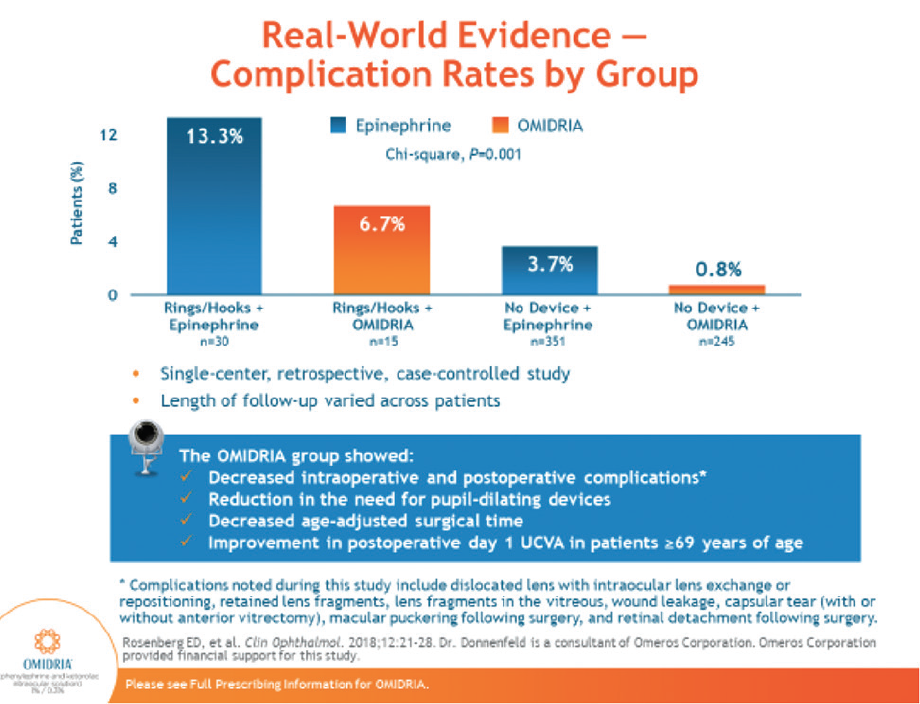
Figure 3. Compared to epinephrine, OMIDRIA is proven to decrease intraoperative and postoperative complications.
Ms. Barkey: We’re now looking at how to elevate quality for the Merit-Based Incentive Payment System (MIPS). We utilize OMIDRIA to produce better quality of care, better outcomes, and fewer complications. With OMIDRIA in our center, cases don’t take as long, and we have fewer complications and return visits to the OR. At the end of the day, we’re saving money, which makes our centers look better. In the real world, I think that the outcomes are going to help make our centers the most revered facilities by insurance companies.
From a surgeon’s perspective, all our younger surgeons want the drug, and we want to do whatever we can to make cases less stressful for them.22 And our senior physician is all about OMIDRIA right now.
Managing Miosis During Femtosecond Surgery
Dr. Lindstrom: What’s your experience with OMIDRIA and the femtosecond laser?
Dr. Silverstein: In a femtosecond procedure, many patients develop ‘femto-miosis’. Mild external pressure on the eye from the laser interface, which inflates intraocular pressure, causes the pupil to constrict. OMIDRIA is particularly helpful in these cases, which occur equally among men and women, because it helps maintain dilation after the pupil has come down from the femtosecond laser.20,23
Dr. Visco: In a standard cataract procedure, OMIDRIA is the first thing the patient receives as we start the surgery. I make a side-port incision and immediately put the BSS solution with OMIDRIA into the eye to start the case. In a femtosecond procedure, the pupil is, of course, dilated for the femtosecond laser. If we have miosis after the femto treatment, as soon as we put OMIDRIA in the eye, the pupil opens right up, and it’s very unusual to encounter problems.
Dr. Lindstrom: I think it’s a clinical pearl. Preoperatively, I use phenylephrine and maybe a topical NSAID to try to reduce femtosecond miosis. OMIDRIA is up in the BSS bottle with much more than we need, so I draw a little up in a syringe and inject it through a side port as a first step. Do you think OMIDRIA works as well or better than epinephrine when used the same way?
Dr. Walter: Sometimes the iris covers the capsulotomy because treatment is causing prostaglandin release. When the patient reaches the OR five minutes later, the pupil has shrunk. We published a study in a peer-reviewed journal comparing epinephrine to OMIDRIA in 100 consecutive femtosecond cataract cases each. We put in OMIDRIA right at the beginning through a side port. We saw just 2% use of Malyugin Rings with OMIDRIA compared to 12% with epinephrine. Surgical time with OMIDRIA was significantly shorter at 8.1 minutes versus 9.4 minutes with epinephrine. OMIDRIA made a big difference.20
Reducing Postoperative Anti-inflammatory Drops
Dr. Lindstrom: OMIDRIA is shown to improve uncorrected visual acuity on day 1, compared to epinephrine.18 That’s certainly a patient satisfaction issue. It also helps deliver NSAID to the anterior chamber to inhibit COX-1 and COX-2 enzymes.24,25 I use just an NSAID drop postoperatively with no steroid. Some surgeons are resistant to that idea because they’ve used steroids for decades, but I think the NSAID is a better drug for postoperative inflammation.
Dr. Walter: It’s really about inhibiting COX-1 and COX-2 enzymes to reduce or shut down prostaglandin formation. Steroids do a poor job because they have to take a circuitous route that depends on cell penetration and patient compliance.26,27 To reduce the compliance factor, I’ve been using only an NSAID after cataract surgery for 8 years, even in some complex cases with diabetic patients or use of pupil-expanding devices. I like that with OMIDRIA during surgery, we can clearly see that we don’t need a postoperative steroid. I think that relates directly to the shutdown of COX-1 and COX-2.
Dr. Visco: Another issue with both NSAIDs and steroids is cost to the patient. They’ve gone through the roof. I dropped steroids 3 or 4 years ago to get those costs down. When OMIDRIA came on the market, I was satisfied that OMIDRIA’s ability to control pain would be effective alongside a postoperative NSAID without a steroid. My patients have not shown a higher incidence of problems. When we did a retrospective study of patients’ charts, looking at those who had an NSAID/steroid combination versus those who received NSAID/OMIDRIA, the NSAID/OMIDRIA group actually fared much better, with less pain and photophobia, less breakthrough iritis, and less CME.28
Using Fewer Pupil-Expanding Devices
Dr. Lindstrom: In a study comparing OMIDRIA and epinephrine, with and without rings, the OMIDRIA group showed decreased intraoperative and postoperative complications, reduced need for pupil-expanding devices, decreased surgical time, and improvement in visual acuity the day after surgery.18 Several other studies have also shown reduced use of pupil-expanding devices.12,19,21
I haven’t been a big advocate of pupil-expanding devices, and I don’t use them at all since I started using OMIDRIA, and I think this is a significant finding. What does it mean in the ASC environment? These are expensive devices, they add time, and there may be a meaningful advantage in avoiding a device. What do you think?
Ms. Barkey: This is a big advantage for us. To the ASC staff, this is meaningful because they experience the frustration in the OR and the pressure to keep costs consistent and justified.
Dr. Silverstein: It’s beneficial for three reasons: First, it saves time, and time in the OR is associated with a variety of different opportunities, including reduced infection risk from spending less time in the eye. Number two, a device can permanently disfigure the iris and pupil structure through micro tears, even with very delicate placement.2 Finally, the pupil-expanding device adds significant cost, which is not reimbursed to the surgery center. If we can reduce cost while enhancing safety and helping ensure the desired outcomes, then the opportunity to use these devices less frequently is very important.
Dr. Visco: We looked at use of pupil-expanding devices with OMIDRIA retrospectively (Figure 4).12 We had been following our standard protocol, marking charts for patients who might potentially need a device so we would know how many to stock in advance. When we looked back at OMIDRIA patients, we found that we hadn’t used any of the rings we’d put in reserve. Now we don’t have to stock as many of these devices, which has made a big difference for our surgery center.
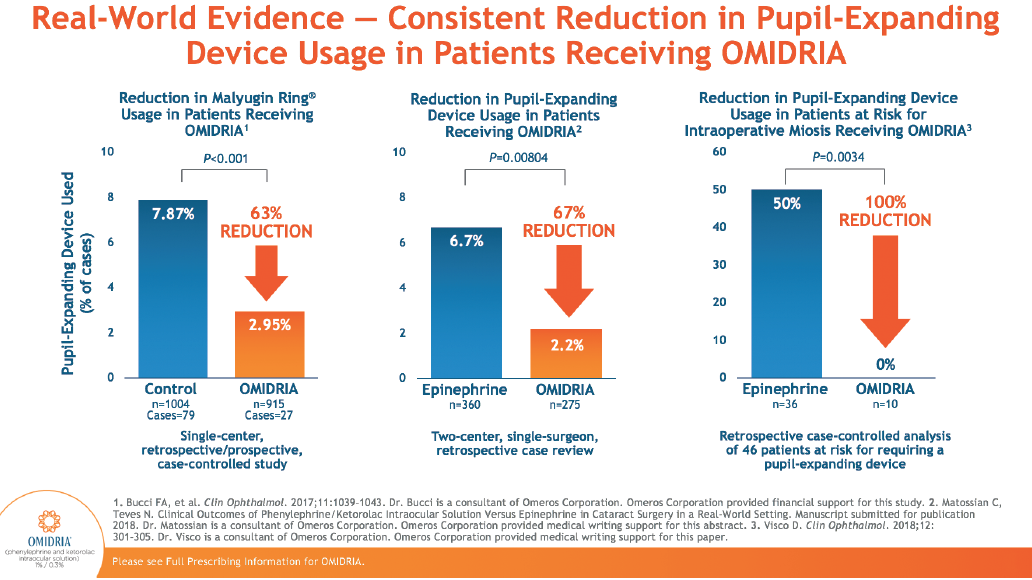
Figure 4. OMIDRIA reduces use of pupil-expanding devices compared to epinephrine.
Dr. Walter: In our paper, we saw about an 80% reduction in using pupil-expanding devices for femtosecond cases, and I find the numbers are similar for regular cases as well.20 Being in an academic medical center, we face concern about costs on a case-by-case basis. The rings are expensive, the ASC or hospital absorbs that cost, and any added code that benefits the surgeon does not reach the facility. OMIDRIA’s ability to reduce device use makes it a positive addition to the hospital setting.
Dr. Lindstrom: We hear that OMIDRIA reduces OR time. Why do you think that difference exists?
Ms. Barkey: We monitor OR times and, when a case takes longer, it’s always more complex. I think OMIDRIA shortens OR time by preventing cases from becoming complex. We need to consider that as we look for quality outcomes, as well as reimbursement and case volume.
Dr. Visco: When a pupil isn’t well-dilated and a complication occurs, such as a tear in the capsular bag, it’s more difficult to see the problem and take care of it. It’s like driving–we have to go slowly when we can’t see well.
Reducing Complications
Dr. Lindstrom: OMIDRIA is the only FDA-approved intraocular product that inhibits prostaglandin release, thus maintaining iris tone, blocking miosis, and preventing IFIS.16,17 In a post-marketing study, OMIDRIA reduced IFIS symptoms by 83%, iris prolapse by 79%, and severe billowing by 90%.16,17 How important are these differences?
Dr. Silverstein: Every surgeon experiences the complications of IFIS—the triad of pupillary miosis, prolapse of the iris through either of the surgical wounds, and flaccidity of the iris. It makes surgery more difficult, with more potential for complications. In a double-blinded, randomized study, we showed a tremendously significant reduction of all three components of IFIS with OMIDRIA in patients exposed to tamsulosin.17 Pupils maintained dilation of 6 mm or more from start to finish—much greater than the group without OMIDRIA. It was the first time we’ve ever demonstrated any way to significantly reduce the impact of exposure to tamsulosin.
Dr. Walter: In Dr. Silverstein’s study, he was able to measure significant reductions in billowing and iris prolapse with OMIDRIA.17 When my students say, ‘I don’t need OMIDRIA. I’m a good surgeon,’ I tell them that all good surgeons can do better. Getting 79% less iris prolapse is a lot better. You may not think iris prolapse is a big deal, but I get referred cases for corneal tattoo because of temporal iris defects all the time.29,30 These patients are miserable and OMIDRIA could potentially prevent this.
Dr. Lindstrom: Dr. Visco, you compared the incidence of CME, breakthrough iritis, and postoperative pain and photophobia in cases with OMIDRIA and topical NSAID versus topical steroid and NSAID with no OMIDRIA. Tell us about the study.
Dr. Visco: It was a retrospective study of 2,277 cases over the course of a period where we changed our perioperative regimen from topical steroid and NSAID to OMIDRIA and topical NSAID.31 The study encompassed 895 patients with the old protocol and 1,382 with the new protocol including OMIDRIA. The incidence of CME in patients with no history of vitreomacular traction syndrome was three times lower with OMIDRIA. In postoperative visits, we noted a two-fold reduction in breakthrough iritis and three times lower incidence of pain and photophobia. We are now looking at the data in much more detail to establish clinical significance and eliminate any bias.
Dr. Lindstrom: How many patients in this study required rescue with a topical steroid?
Dr. Visco: In both groups, we did have to rescue a small percentage of patients with steroid.31 The OMIDRIA group required steroid about half as many times: 2.9% versus 4.7% in the steroid group. We would add on or change up the topical steroid for both breakthrough iritis or unexplained onset of photophobia (Figure 5). Loteprednolol was our standard postoperative topical steroid in the control group, and for rescue in either group we would use Pred Forte, Durazol, or possibly just restart the Lotemax gel.
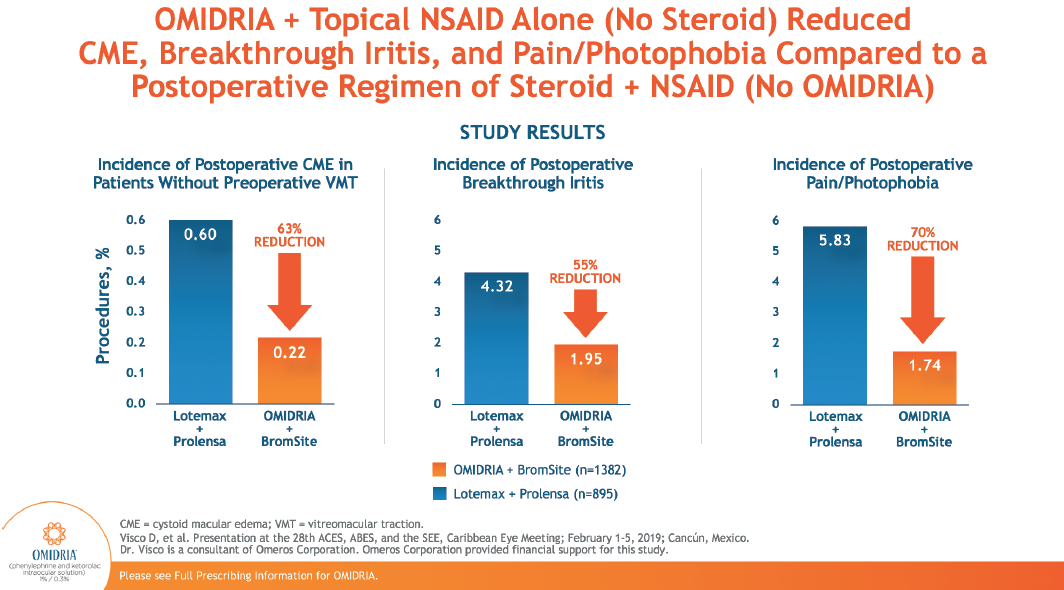
Figure 5. OMIDRIA is proven to reduce incidence of postoperative CME, rebound iritis, pain, and photophobia.
My goal in dropping steroids 3 to 4 years ago was to get the costs down for the patient. When OMIDRIA came on the market and I started using it, I saw such great results in surgery and I thought, let’s try this with just the NSAID postoperatively. I thought combining OMIDRIA with a topical NSAID should do the trick, and I kind of crossed my fingers and held my breath. You know what? My practice ran better than ever. I did not have a waiting room full of breakthrough iritis and pain because I dropped the steroid. I saw less of these patients which is good for both them and me.
Dr. Walter: It’s really about inhibiting COX-1 and COX-2 enzymes to reduce or totally shut down prostaglandin formation. Steroids do a poor job of doing that because they rely on compliance and penetration into the cell. NSAIDs penetrate more easily. It turns out we really don’t need a steroid in most cataract cases.
In the internal data at our practice, steroid rescue is used in about 1.5% to 2% of cases. These are typically cases where there is a little retained cortex, we did a lot of iris manipulation, or there was an extenuating circumstance that resulted in a longer surgery. These patients experience inflammation longer and need a bit more control.
Dr. Silverstein: In our practice, we see breakthrough or persistent iritis in about 3% to 5% of cases. The groups that most frequently need steroid rescue are people of African American or Hispanic descent, or people who are at risk for or have a history of iritis. Diabetes is a soft risk factor as well, because it’s a disease that affects blood vessels, and uveitis has a different severity profile. They need a strong steroid, plus or minus an NSAID, and I use both. Some patients may also need a periocular injection if they are not known steroid responders, especially if they have CME.
Dr. Lindstrom: How do postoperative visits from pain, inflammation, and CME affect the clinic?
Ms. Barkey: We don’t want that extra chair time in our clinic, and I don’t think any physician does. It absolutely matters to us. It also affects patients’ perception of surgery and the practice. We want patients to have a positive experience and tell their friends.
Dr. Lindstrom: Dr. Walter, you did a retrospective study of published research about the effect of OMIDRIA on the incidence of CME. Tell us about it.
Dr. Walter: We just looked at one outcome: the incidence of CME. We eliminated previous retinal pathology and looked at 504 eyes in 357 patients.32 We found only two cases of postoperative CME in patients who had OMIDRIA. The threshold for detecting CME was 20/30 vision or vision that was worse 6 weeks after surgery. CME was detected using OCT. We found that incidence of CME was reduced 3- to 15-fold by the use of OMIDRIA, as compared to historical published studies (Figure 6).
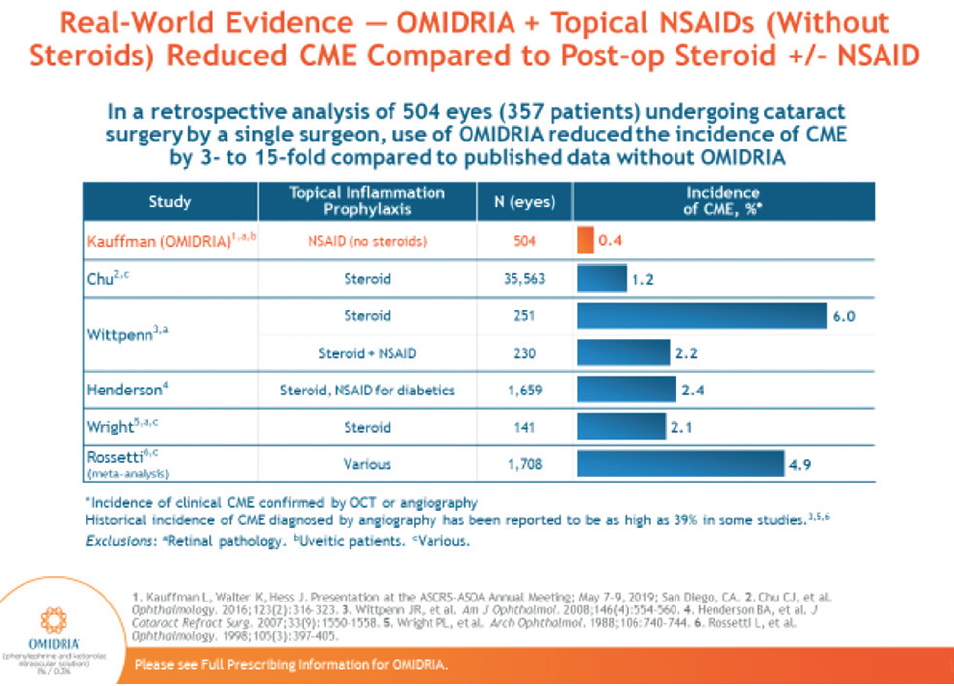
Figure 6. Use of OMIDRIA + topical NSAID is shown to reduce the incidence of CME compared to postoperative steroids.
Experiences with OMIDRIA
Dr. Lindstrom: Do you use OMIDRIA selectively or for all your patients?
Dr. Silverstein: I use OMIDRIA in every case. It’s not just for complex or at-risk patients. Every patient has inflammation as a result of even our most stealth surgery, so even if a patient will not have a problem with pupil dilation, I want to get OMIDRIA’s anti-inflammatory effect. I want to take the opportunity to hit inflammation hard—at its source and at the time that we’re creating it. When I have my own surgery someday, I’m going to strongly request that my surgeon has OMIDRIA in the irrigant.
Using OMIDRIA for every case also makes it routine, and every surgeon loves routine. The stress of surgery can be significant. Routine makes a surgical day much more enjoyable and less stressful. It’s very practical to use it for every case as well. From the ASC’s standpoint, it creates no time-consuming burden for staff. We explain the benefits, the staff members understand it, and it takes no extra time to inject it into the irrigation solution as we’re setting up the room.
Dr. Walter: For the first year after OMIDRIA was approved, I was a naysayer because of the perceived cost, or my misunderstanding of the pass-through payment system. Then I tried it on about 30 patients, and I became convinced it had clear clinical benefits. We started using it during the first pass-through period, and now that it’s back under pass-through status, we’re using it in about 70% to 80% of cases.
Ms. Barkey: In our facility, we’ve been using OMIDRIA for about 2 years, and we try to use it on every patient. In our clinic, when we’re verifying coverage for surgery, we also verify the CPT code for OMIDRIA. That way our surgeons know right away which patients have access to OMIDRIA. Surgeons want OMIDRIA to be available to them—it’s something they’re looking for in a facility.
Dr. Lindstrom: When you talk to peers who aren’t using OMIDRIA and they say it doesn’t make sense to them, what do you say?
Dr. Visco: OMIDRIA is one of the best tools I have. It makes my operating day go very smoothly and pleasantly, and I get good outcomes for my patients. I get to go home with a nice smile on my face because I very rarely have any complications.
Dr. Lindstrom: Dr. Walter, what do you tell your colleagues, as someone who went from a naysayer to a ‘yaysayer’?
Dr. Walter: I tell my conversion story and suggest that they try it. For me, it took about 30 cases to be convinced. What really stood out were fellow eye procedures after I’d had a difficult time with the patient’s first eye without OMIDRIA. Even if our peers start using OMIDRIA for patients who have IFIS or risk factors for miosis, I think they’ll quickly have a different feeling about it.
Dr. Visco: I love your story, Dr. Walter, because when OMIDRIA was first available, everybody was talking about it. There was a big controversy concerning the cost and the pass-through. There were a lot of people on both sides, and I was so intrigued I had to use it and see for myself if it worked. After all, it’s the patient that matters most in this whole story. I think that’s a big message for all of us. If we see something that’s FDA approved, has an adverse event profile that’s comparable to placebo,16 and should be good for patients, why not try it yourself? Just try it and formulate your own opinion about how it works.
Ms. Barkey: One of our esteemed physicians who has performed cataract surgery for decades asked why we would need to add the expense of OMIDRIA when surgery was largely quite smooth. Now he sees that it actually takes some stress off of him during surgery. I think that’s a big message for surgeons.
Dr. Silverstein: Omeros is a pioneer. The company is helping doctors understand what this medicine is all about because there was nothing like it in the past. This is completely novel. I recommend that my peers try it themselves.
Patient Selection for OMIDRIA
Dr. Lindstrom: You mentioned recommending that peers try using OMIDRIA with the patients who need it most, perhaps for dilation. What are the characteristics of those patients?
Dr. Silverstein: Patients that benefit more are definitely those with IFIS and those who have had unexpected pupil constriction. Pupil size can come down during surgery even without tamsulosin exposure, but patients taking this medication have a higher risk of IFIS, as do those who have a history of IFIS in the fellow eye. Anyone who is on the high-risk list with past history of vitreoretinal procedures, cardiovascular disease, prior history of uveitis, or diabetes can also get particular benefit from the medicine.
Dr. Walter: We went through an exercise with the cataract surgeons at Wake Forest where we came up with a group of indications to use internally for OMIDRIA. The idea was that the indications would put us all on the same page if MIPS became a concern in the future. Number one was femtosecond cataract surgery, followed by IFIS or history of IFIS in the other eye, a resident performing the procedure, suboptimal dilation in the clinic (less than 6 mm), and history of alpha blocker.
Dr. Lindstrom: Do you worry about a history of NSAID allergy? What do you think of the adverse event profile in general?
Dr. Walter: I always ask patients to describe their NSAID allergy because, while that might only mean heartburn or gastrointestinal upset, what concerns me is anaphylaxis and rash. If they’ve had anaphylaxis and rash, then of course we don’t use OMIDRIA, but if someone has had stomach upset from a previous NSAID, I do use it. In our practice, we haven’t seen any adverse effects related to NSAID allergy.
When we talk about adverse events, we need to consider OMIDRIA versus compounding agents, because that was a key to convincing our hospital to use OMIDRIA. They’re all very much against compounding after some of the headline situations where non-sterile products were used in the eye, causing toxic anterior segment syndrome, endophthalmitis, and even blindness. OMIDRIA is a very welcome and proven alternative.
Reimbursement for OMIDRIA
Dr. Lindstrom: OMIDRIA has broad and expanding coverage and reimbursement through Medicare, Medicare Advantage, Medicaid, and commercial payers. Omeros also has the OMIDRIAssure Patient Assistance and Reimbursement Program to help patients with and without coverage gain access to OMIDRIA. In addition, pass-through reimbursement was reinstated for OMIDRIA through October 1, 2020. Do you feel like you’re benefitting from increased coverage of OMIDRIA and its pass-through reimbursement?
Dr. Silverstein: With over 2,000 OMIDRIA cases under our belt, we get a lot of calls from practices asking our staff how they should approach OMIDRIA reimbursement for the first time. By now, it’s second nature to our staff. It’s as simple as precertifying a patient for surgery so, from the staff’s point of view, it’s a very streamlined and easy process. That’s important because there are medicines and devices we use, such as pupil-expanding devices, that require huge financial outlays that are never recovered by the surgical center. It’s reassuring that the surgery center is reimbursed for OMIDRIA, so it doesn’t cost the surgery center or its owners.
Most ASCs do not have the personnel to obtain prior authorization or go through the qualification process. Since our practice does have a staff that is trained to do precertifications and submit for authorization of a variety of procedures, we took the time burden away from the ASC and brought it easily in house within the practice. This was a huge relief for the ASC staff.
Dr. Visco: The physicians that operate at our center were very happy for the opportunity to use OMIDRIA, and that’s possible because it is reimbursed. We have not had difficulties with coverage. My staff is pretty meticulous about following the rules. If we can’t get reimbursement for a patient, then we work with available assistance programs. It’s been a good experience for us. My advice for any facility that’s doing this would be to have a designated person that works on all of the practice’s OMIDRIA reimbursement. We have one person in charge, and that person makes sure we’re not falling behind with accounts receivable.
Dr. Walter: The pass-through has been very positive for us. In every case where we’ve used it, we’ve gotten paid. In our hospital, we needed to get the pharmacists to buy in to the pass-through process. They have incentives to reduce costs, and we explained that although this is a large item in their budget, the hospital will get paid for it. We reinforced that reassurance by bringing administrators into the discussion. Omeros helped by sending representatives to explain the process at our institution. Now everyone is on board. It’s a positive experience for everybody because the pharmacists aren’t worried about problems with Medicare, billing patients, or sticking the hospital with the bill. They see that it’s actually working. At the end of the day, though, it’s our patients who benefit from pass-through payment for OMIDRIA—access to the drug means better patient outcomes.
Ms. Barkey: We watch the OMIDRIA claims, and we’ve had no issue with claim denials as long as we do our due diligence up front. The availability of a permanent, product-specific J-code for OMIDRIA (J1097) has streamlined billing (Figures 7 and 8). We use the staff training that Omeros provides, and we’ve created a protocol for our practice and ASC to make sure we’re doing things right. We’ve got until October 1, 2020 to utilize the pass-through program as much as we possibly can and prove that this is working for our patients to deliver high quality outcomes.

Figure 7. Unique, permanent J-code for OMIDRIA is J1097.
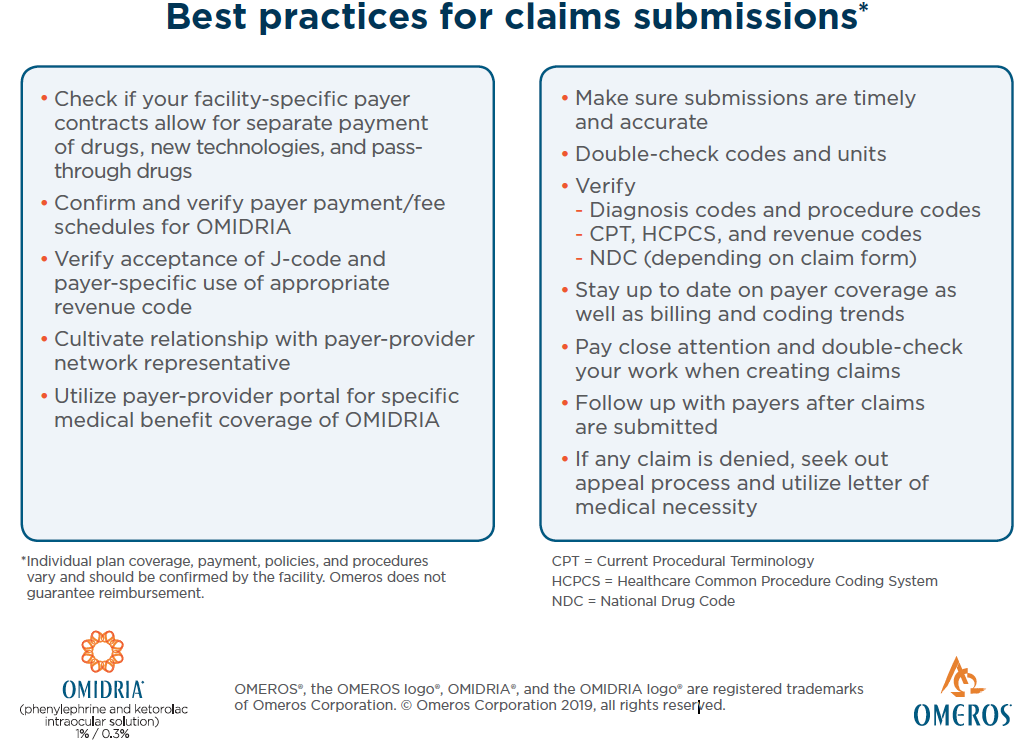
Figure 8. Claims submissions best practices.
How Has OMIDRIA Changed Cataract Surgery?
Dr. Lindstrom: Finally, how has OMIDRIA changed your cataract surgery experience from a clinical or an administrative perspective? What keeps you advocating for OMIDRIA, both for your patients and yourself?
Dr. Silverstein: In the past, I never had anything that could consistently help a patient at risk for IFIS, but I now have that tool, and that’s huge. And it has helped me create a happier patient in the early postoperative phase because they experience less pain. In the majority of cases, the inflammation has cleared and vision is 20/20 or better. OMIDRIA has helped us get there sooner, and today that’s the name of the game.
Dr. Visco: It’s so important for physicians to be involved and to make their voices heard about what’s good for the patients. In my previous facility, I could not use OMIDRIA. Now I have my own center and I can have what I want, but not everyone is in that situation. As much as we can, we need to advocate for what’s best for our patients and the care we want to deliver as surgeons. Ultimately, this is about physicians being able to provide—and patients being able to access—the best medical care. I’m very happy that the research we have done showing the benefits of OMIDRIA has made it possible for some surgeons to access the product that were previously locked out.
OMIDRIA has changed my cataract surgery experience for the better. I enjoy my surgery days much more, and I don’t anticipate as many problems. I think using OMIDRIA has even potentially increased my longevity as a surgeon by decreasing my stress level in the OR. With the aging population and the tsunami of baby boomers we expect to see for cataract surgery, we will likely have a shortage of physicians, which would be a serious problem for the public. The things that make my job easier may also push off my retirement a little bit, so I can continue to serve patients longer and personally enjoy my practice, too.
Dr. Walter: My experience using OMIDRIA for cataract surgery is about consistency. When I have OMIDRIA on board, I have a consistent, easy day in surgery where my cases proceed very predictably. That gives me comfort, and I think my patients sense that I’m confident and relaxed. I see the same consistency in postoperative outcomes. My clinic runs smoothly, patients are happy, and everybody has clear corneas and good vision. That’s how OMIDRIA has changed my surgical experience and set me apart from doctors who don’t use it.
Ms. Barkey: As an administrator, I see faster surgery. Surgical staff members are happier because we can control patient flow and efficiency and predict how our day is going to go. OMIDRIA contributes to that speed, predictability, and positive atmosphere.
Dr. Silverstein: OMIDRIA’s biggest impact occurs with our patients. Patients want to feel comfortable, see clearly, and return to normal activities as soon as possible. Anything we can do to interfere with COX-1 and COX-2 enzymes releasing prostaglandins, thus controlling inflammation as it begins, will influence how quickly the patient heals. We see corneal clarity and low subjective pain scores. This is very demonstrable.
I highly encourage each of my colleagues to trial OMIDRIA, not just in their at-risk patients or IFIS patients, but also in their low-risk, uncomplicated patients. They will see for themselves that this medicine truly is a benefit to patients and to the surgical experience.
1. Cho H, Wolf KJ, Wolf EJ. Management of ocular inflammation and pain following cataract surgery: focus on bromfenac ophthalmic solution. Clin Ophthalmol. 2009;3:199-210.
2. Al-Hashimi S, Donaldson K, Davidson R, et al. Medical and surgical management of the small pupil during cataract surgery. J Cataract Refract Surg. 2018;44(8):1032-1041.
3. DeStafeno JJ, Kim T. Perioperative and postoperative medications. Essentials of Cataract Surgery. 2014:207-217.
4. Fung D, Cohen MM, Stewart S, et al. What determines patient satisfaction with cataract care under topical local anesthesia and monitored sedation in a community hospital setting? Anesth Analg. 2005;100(6):1644-1650.
5. Porela-Tiihonen S, Kaarniranta K, Kokki H. Postoperative pain after cataract surgery. J Cataract Refract Surg. 2013;39(5):789-798.
6. Akman A, Yilmaz G, Oto S, et al. Comparison of various pupil dilatation methods for phacoemulsification in eyes with a small pupil secondary to pseudoexfoliation. Ophthalmology. 2004;111(9):1693-1698.
7. Olson RJ, Braga-Mele R, Chen SH, et al. Cataract in the Adult Eye Preferred Practice Pattern. Ophthalmology. 2017;124(2):P1-P119.
8. Chang DF, Campbell JR, Colin J, et al. Prospective masked comparison of intraoperative floppy iris syndrome severity with tamsulosin versus alfuzosin. Ophthalmology. 2014;121(4):829-834.
9. Zaczek A, Zetterström C. Cataract surgery and pupil size in patients with diabetes mellitus. Acta Ophthalmol Scand. 1997;75(4):429-432.
10. Megbelayin EO, Pindikura S. Managing challenges of recalcitrant intra-operative miosis during small incision cataract surgery. Int J Sci Res Knowledge. 2013;1:74-81.
11. Bäckström G, Behndig A. Redilatation with intracameral mydriatics in phacoemulsification surgery. Acta Ophthalmol Scand. 2006;84(1):100-104.
12. Visco D. Effect of phenylephrine/ketorolac on iris fixation ring use and surgical times in patients at risk of intraoperative miosis. Clin Ophthalmol. 2018;12:301-305.
13. Lundberg B, Behndig A. Intracameral mydriatics in phacoemulsification surgery obviate the need for epinephrine irrigation. Acta Ophthalmologica Scandinavica. 2007;85:546-551.
14. U.S. Illnesses and deaths associated with compounded medications or repackaged medications. pewtrusts.org. http://www.pewtrusts.org/en/research-and-analysis/data-visualizations/2017/us-illnesses-and-deaths-associated-with-compounded-medications-or-repackaged-medications. Accessed August 28, 2019.
15. Department of health and human services food and drug administration. fda.gov. https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-orgs/documents/document/ucm572852.pdf. Accessed August 28, 2019.
16. OMIDRIA [package insert]. accessdata.fda.gov. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205388s000lbl.pdf. Accessed August 28, 2019.
17. Silverstein SM, Rana VK, Stephens R, et al. Effect of phenylephrine 1.0%-ketorolac 0.3% injection on tamsulosin-associated intraoperative floppy-iris syndrome. J Cataract Refract Surg. 2018;44(9):1103-1108.
18. Rosenberg ED, Nattis AS, Alevi D, et al. Visual outcomes, efficacy, and surgical complications associated with intracameral phenylephrine 1.0%/ketorolac 0.3% administered during cataract surgery. Clin Ophthalmol. 2017;12:21-28.
19. Bucci FA Jr, Michalek B, Fluet AT. Comparison of the frequency of use of a pupil expansion device with and without an intracameral phenylephrine and ketorolac injection 1%/0.3% at the time of routine cataract surgery. Clin Ophthalmol. 2017;11:1039-1043.
20. Walter K, Delwadia N, Coben J. Continuous intracameral phenylephrine-ketorolac irrigation for miosis prevention in femtosecond laser-assisted cataract surgery: reduction in surgical time and iris manipulation. J Cataract Refract Surg. 2019;45(4):465-469.
21. Matossian C, Teves N. Clinical outcomes of phenylephrine/ketorolac intraocular solution versus epinephrine in cataract surgery in a real-world setting. Manuscript submitted for publication. 2018.
22. Omeros survey data on file.
23. Gayton JL. Effect of early phenylephrine and ketorolac injection 1%/0.3% (Omidria) usage on pupil diameter in traditional and femto-assisted cataract surgery. Presented at: 15th International Congress on Vision Science and Eye; Aug. 10-11, 2017, London, United Kingdom.
24. Waterbury LD. Alternative drug delivery for patients undergoing cataract surgery as demonstrated in a canine model. J Ocular Pharmacol Ther. 2018;34(1-2):154-160.
25. Katsev DA, Katsev CC, Pinnow J, et al. Intracameral ketorolac concentration at the beginning and end of cataract surgery following preoperative topical ketorolac administration. Clin Ophthalmol. 2017;11:1897-1901.
26. Stevens CE, Bennion JL, Caldwell MC, et al. Dose uniformity of topical corticosteroids: a simulated trial of fluorometholone acetate 0.1% and loteprednol etabonate gel 0.5. J Ocul Pharmacol Ther. 2017;33(2):111-114.
27. Apt L, Henrick A, Silverman LM. Patient compliance with use of topical ophthalmic corticosteroid suspensions. Am J Ophthalmol. 1979;87(2):210-214.
28. Visco D, et al. Study to evaluate patient outcomes following cataract surgery when using OMIDRIA with postoperative topical NSAID administration versus a standard regimen of postoperative topical NSAIDs and steroids. Presented at: 28th Annual Meeting of the American College of Eye Surgeons (ACES), the American Board of Eye Surgery (ABES), and the Society for Excellence in Eyecare (SEE), Caribbean Eye Meeting; February 1-5, 2019, Cancún, Mexico.
29. Reed JW. Corneal tattooing to reduce glare in cases of traumatic iris loss. Cornea. 1994;15(5):401-405.
30. Alio JL, Rodriguez AE, Toffaha BT. Keratopigmentation (corneal tattooing) for the management of visual disabilities of the eye related to iris defects. Br J Ophthalmol. 2011;95(10):1397-1401.
31. Visco D. Be prepared to expect the unexpected. Presentation at: 28th ACES, ABES, and the SEE, Caribbean Eye Meeting; February 1-5, 2019, Cancún, Mexico.
32. Walter K, Kauffman L, Hess J. Rate of pseudophakic cystoid macular edema using intraoperative and topical NSAIDs alone without steroids. Manuscript submitted for publication. 2019.
INDICATIONS AND USAGE
OMIDRIA® is added to ophthalmic irrigating solution used during cataract surgery or intraocular lens replacement and is indicated for maintaining pupil size by preventing intraoperative miosis and reducing postoperative ocular pain.
IMPORTANT SAFETY INFORMATION
OMIDRIA must be added to irrigating solution prior to intraocular use.
OMIDRIA is contraindicated in patients with a known hypersensitivity to any of its ingredients.
Systemic exposure of phenylephrine may cause elevations in blood pressure.
Use OMIDRIA with caution in individuals who have previously exhibited sensitivities to acetylsalicylic acid, phenylacetic acid derivatives, and other nonsteroidal anti-inflammatory drugs (NSAIDs), or have a past medical history of asthma.
The most commonly reported adverse reactions at ≥ 2% are eye irritation, posterior capsule opacification, increased intraocular pressure, and anterior chamber inflammation.
Please see the Full Prescribing Information for OMIDRIA.
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OMIDRIA® safely and effectively. See full prescribing information for OMIDRIA.
OMIDRIA® (phenylephrine and ketorolac intraocular solution) 1% / 0.3%, for addition to ocular irrigating solution
Initial U.S. Approval: 2014
INDICATIONS AND USAGE
OMIDRIA is an alpha 1-adrenergic receptor agonist and nonselective cyclooxygenase inhibitor indicated for:
• Maintaining pupil size by preventing intraoperative miosis (1)
• Reducing postoperative pain (1)
OMIDRIA is added to an ocular irrigating solution used during cataract surgery or intraocular lens replacement.
DOSAGE AND ADMINISTRATION
• Each vial of OMIDRIA must be diluted prior to use for administration to a single patient undergoing cataract surgery or intraocular lens replacement.
• Dilute 4 mL of OMIDRIA in 500 mL of ocular irrigating solution. Irrigation solution is to be used as needed for the surgical procedure. (2)
DOSAGE FORMS AND STRENGTHS
Intraocular solution containing phenylephrine 10.16 mg/mL (1%) and ketorolac 2.88 mg/mL (0.3%) for use in a single patient. (3)
CONTRAINDICATIONS
Hypersensitivity to any component of this product (4)
WARNINGS AND PRECAUTIONS
Systemic exposure to phenylephrine may cause elevations in blood pressure. (5.1)
ADVERSE REACTIONS
The most common reported adverse reactions (≥2%) are eye irritation, posterior capsule opacification, increased intraocular pressure, and anterior chamber inflammation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Omeros Corporation at 1-844-OMEROS1 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION
Revised: 12/2017
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Elevated Blood Pressure
5.2 Cross-Sensitivity or Hypersensitivity
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
*Sections or subsections omitted from the full prescribing information are not listed
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Omidria® is added to an ocular irrigating solution used during cataract surgery or intraocular lens replacement and is indicated for maintaining pupil size by preventing intraoperative miosis and reducing postoperative ocular pain.
2 DOSAGE AND ADMINISTRATION
Omidria must be diluted prior to intraocular use. For administration to patients undergoing cataract surgery or intraocular lens replacement, 4 mL of Omidria is diluted in 500 mL of ocular irrigating solution. Irrigation solution is to be used as needed for the surgical procedure for a single patient.
The storage period for the diluted product is not more than 4 hours at room temperature or 24 hours under refrigerated conditions.
Do not use if the solution is cloudy or if it contains particulate matter.
3 DOSAGE FORMS AND STRENGTHS
Omidria is an intraocular solution containing 10.16 mg/mL (1% w/v) of phenylephrine and 2.88 mg/mL (0.3% w/v) of ketorolac for use in a single patient.
4 CONTRAINDICATIONS
Omidria is contraindicated in patients with a known hypersensitivity to any of its ingredients.
5 WARNINGS AND PRECAUTIONS
5.1 Elevated Blood Pressure
Systemic exposure to phenylephrine can cause elevations in blood pressure.
5.2 Cross-Sensitivity or Hypersensitivity
There is the potential for cross-sensitivity to acetylsalicylic acid, phenylacetic acid derivatives, and other non-steroidal anti-inflammatory drugs (NSAIDs). There have been reports of bronchospasm or exacerbation of asthma associated with the use of ketorolac in patients who either have a known hypersensitivity to aspirin/NSAIDs or a past medical history of asthma. Therefore, use Omidria with caution in individuals who have previously exhibited sensitivities to these drugs.
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in practice.Table 1 shows frequently reported ocular adverse reactions with an incidence of ≥ 2% of adult patients as seen in the combined clinical trial results from three randomized, placebo-controlled studies [see Clinical Studies (14)].
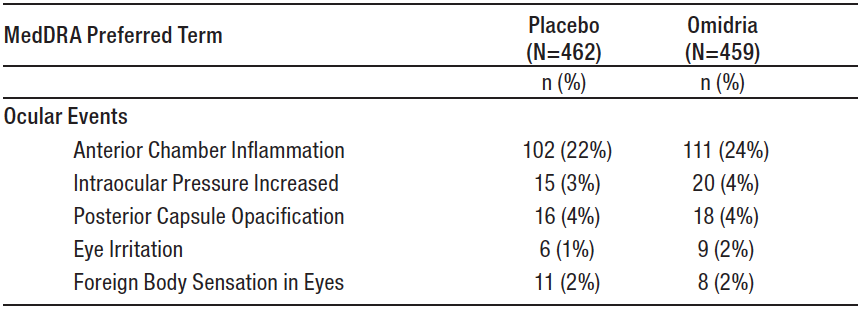
Table 1: Ocular Adverse Reactions Reported by ≥ 2% of Adult Patients
In a safety study that enrolled 72 pediatric patients up to 3 years old, no overall difference in safety was observed between pediatric and adult patients.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on Omidria use in pregnant women or animals to inform any drug-associated risks. Oral administration of ketorolac to rats during late gestation produced dystocia and increased pup mortality at a dose 740-times the plasma exposure at the recommended human ophthalmic dose (RHOD). Since human systemic exposure to Omidria following a lens replacement procedure is low [see Clinical Pharmacology (12.3)], the applicability of animal findings to the risk of Omidria in humans during pregnancy is unclear. Omidria should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Premature closure of the ductus arteriosus in the fetus has occurred with third trimester use of oral and injectable NSAIDs. Ketorolac plasma concentrations are detectable following ocular Omidria administration [see Clinical Pharmacology (12.3)]. The use of Omidria during late pregnancy should be avoided.
Data
Animal Data
No well-controlled animal reproduction studies have been conducted with Omidria or phenylephrine.
Ketorolac, administered during organogenesis, did not cause embryofetal abnormalities or mortalities in rabbits or rats at oral doses of 3.6 mg/kg/day and 10 mg/kg/day, respectively. These doses produced systemic exposure that is 1150 times and 4960 times the plasma exposure (based on Cmax) at the RHOD, respectively. When administered to rats during late gestation (after Day 17 of gestation) at oral doses up to 1.5 mg/kg/day (740 times the plasma exposure at the RHOD), ketorolac produced dystocia and increased pup mortality.
8.2 Lactation
Risk Summary
There are no data on the presence of Omidria in human milk, the effects on the breastfed infant, or the effects on milk production. However, systemic exposure to Omidria, following a lens replacement procedure is low [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Omidria and any potential adverse effects on the breastfed child from Omidria.
8.4 Pediatric Use
The safety and effectiveness of Omidria have been established in the pediatric population from neonates to adolescents (birth to younger than 17 years). Use of Omidria in this population is supported by evidence from adequate and well-controlled studies of Omidria in adults with additional data from a single active-controlled safety study in pediatric patients up to 3 years old [see Clinical Studies (14)].
No overall differences in safety were observed between pediatric and adult patients.
8.5 Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and adult patients.
10 OVERDOSAGE
Systemic overdosage of phenylephrine may cause a rise in blood pressure. It may also cause headache, anxiety, nausea, vomiting, and ventricular arrhythmias. Supportive care is recommended.
11 DESCRIPTION
Omidria is a sterile aqueous solution, containing the α1-adrenergic receptor agonist phenylephrine HCl and the nonsteroidal anti-inflammatory ketorolac tromethamine, for addition to ocular irrigating solution.
The descriptions and structural formulae are:
Phenylephrine Hydrochloride Drug Substance:
Common Name: phenylephrine hydrochloride
Chemical Name: (-)-m-Hydroxy-α-[(methylamino)methyl]benzyl alcohol hydrochloride
Molecular Formula: C9H13NO2 · HCl
Molecular Weight: 203.67 g/mole
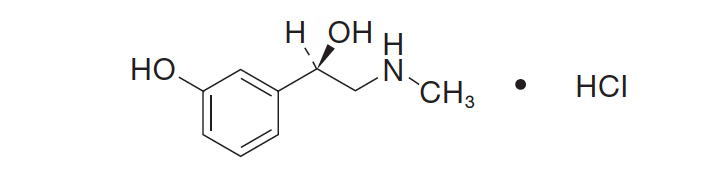
Figure 1: Chemical Structure for Phenylephrine HClHOH
Ketorolac Tromethamine Drug Substance:
Common Name: ketorolac tromethamine
Chemical Name: (±)-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid :2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1)
Molecular Formula: C15H13NO3 · C4H11NO3
Molecular Weight: 376.40 g/mole
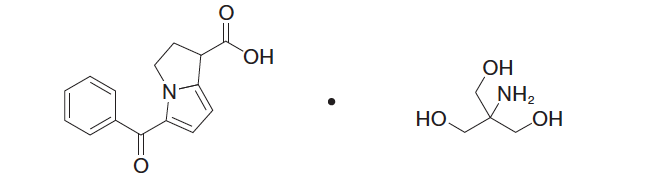
Figure 2: Chemical Structure for Ketorolac Tromethamine
Omidria is a clear, colorless to slightly yellow, sterile solution concentrate with a pH of approximately 6.3.
Each vial of Omidria contains:
Actives:phenylephrine hydrochloride 12.4 mg/mL equivalent to 10.16 mg/mL of phenylephrine and ketorolac tromethamine 4.24 mg/mL equivalent to 2.88 mg/mL of ketorolac.
Inactives:citric acid monohydrate; sodium citrate dihydrate; water for injection; may include sodium hydroxide and/or hydrochloric acid for pH adjustment.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The two active pharmaceutical ingredients (API) in Omidria, phenylephrine and ketorolac, act to maintain pupil size by preventing intraoperative miosis, and reducing postoperative pain.
Phenylephrine is an α1-adrenergic receptor agonist and, in the eye, acts as a mydriatic agent by contracting the radial muscle of the iris. Ketorolac is a nonsteroidal anti-inflammatory that inhibits both cyclooxygenase enzymes (COX-1 and COX-2), resulting in a decrease in tissue concentrations of prostaglandins to reduce pain due to surgical trauma. Ketorolac, by inhibiting prostaglandin synthesis secondary to ocular surgical insult or direct mechanical stimulation of the iris, also prevents surgically induced miosis.
12.3 Pharmacokinetics
In a pharmacokinetic study evaluating Omidria, systemic exposure to both phenylephrine and ketorolac was low or undetectable.
A single-dose of Omidria as part of the irrigation solution was administered in 14 patients during lens replacement surgery. The volume of irrigation solution used during surgery ranged between 150 mL to 300 mL (median 212.5 mL). Detectable phenylephrine plasma concentrations were observed in one of 14 patients (range 1.2 to 1.4 ng/mL) during the first 2 hours after the initiation of Omidria administration. The observed phenylephrine plasma concentrations could not be distinguished from the preoperative administration of phenylephrine 2.5% ophthalmic solution prior to exposure to Omidria.
Ketorolac plasma concentrations were detected in 10 of 14 patients (range 1.0 to 4.2 ng/mL) during the first 8 hours after the initiation of Omidria administration. The maximum ketorolac concentration was 15 ng/mL at 24 hours after the initiation of Omidria administration, which may have been due to application of postoperative ketorolac ophthalmic solution.
14 CLINICAL STUDIES
Studies in Adults
The efficacy and safety of Omidria were evaluated in two Phase 3, randomized, multicenter, double-masked, placebo-controlled clinical trials in 808 adult patients undergoing cataract surgery or intraocular lens replacement.
Patients were randomized to either Omidria or placebo. Patients were treated with preoperative topical mydriatic and anesthetic agents. Pupil diameter was measured throughout the surgical procedure. Postoperative pain was evaluated by self-administered 0-100 mm visual analog scales (VAS).
Mydriasis was maintained in the Omidria-treated groups while the placebo-treated groups experienced progressive constriction.
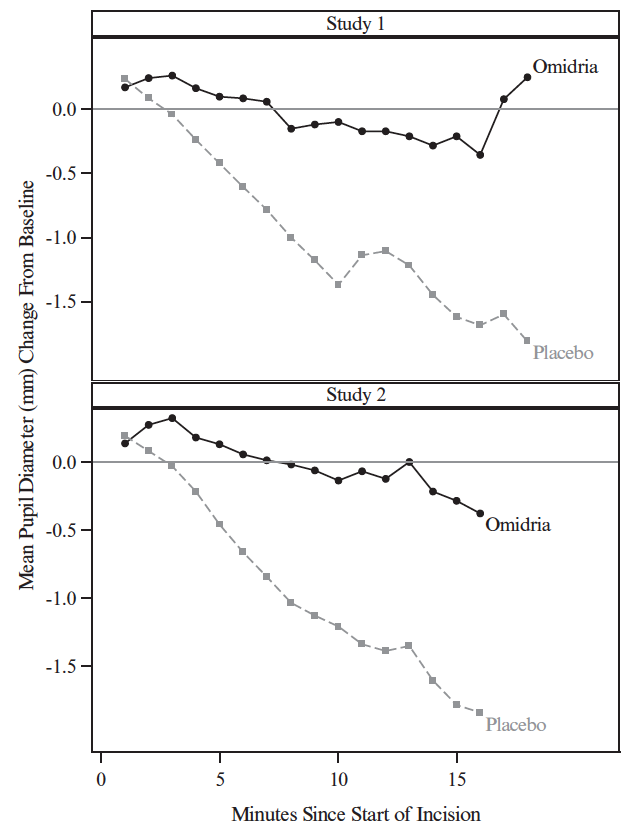
Figure 3: Intraoperative Pupil Diameter (mm) Change-from-Baseline
At the end of cortical clean-up, 23% of placebo-treated patients and 4% of Omidria-treated patients had a pupil diameter less than 6 mm (p < 0.01).
Pain during the initial 10-12 hours postoperatively was statistically significantly less in the Omidria-treated groups than in the placebo-treated groups.
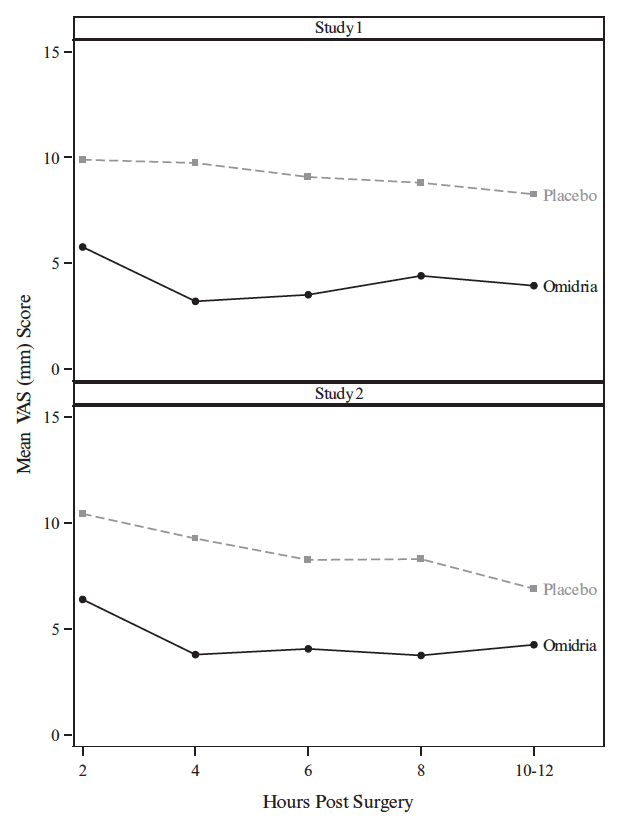
Figure 4: Postoperative Mean Visual Analog Scale (VAS) Scores for Pain
During the 10-12 hours postoperatively, 26% of Omidria-treated patients reported no pain (VAS = 0 at all timepoints) while 17% of placebo-treated patients reported no pain (p < 0.01).
Study in Pediatric Patients
The safety of Omidria was evaluated in a single, randomized, multicenter, double-masked, active-controlled clinical study in 72 pediatric patients up to 3 years old undergoing cataract surgery with or without intraocular lens replacement.
Patients were randomized to either Omidria or phenylephrine. Patients were treated with preoperative topical mydriatic and anesthetic agents. As in the adult studies, mydriasis was maintained in the Omidria-treated group. No overall differences in safety were observed between pediatric and adult patients.
16 HOW SUPPLIED/STORAGE AND HANDLING
Omidria (phenylephrine and ketorolac intraocular solution) 1% / 0.3% is supplied in a clear, 5-mL glass, single-patient-use vial containing 4 mL of sterile solution, for addition to ocular irrigating solution.
Omidria is supplied in a multi-pack containing:
4 vials: NDC 62225-600-04 or
10 vials: NDC 62225-600-10
Storage: Store at 20˚ to 25˚C (68˚ to 77˚F). Protect from light.
17 PATIENT COUNSELING INFORMATION
Inform patients that they may experience sensitivity to light.
Omeros Corporation
201 Elliott Avenue West
Seattle, WA 98119
© Omeros 2013-2017 US Patents 8,173,707, 8,586,633, 9,066,856, 9,278,101, 9,399,040, and 9,486,406; additional patents pending. OMIDRIA® and the OMIDRIA® Logo are registered trademarks of Omeros Corporation. PI100016



