
IOL implantation in the absence of adequate capsular support continues to present challenges for cataract surgeons. Many techniques have been tried, but continued evolution is proof that the holy grail of IOL fixation has yet to be found. I believe, however, that surgeons are getting closer to discovering a technique that can transcend all others previously tried.
Specifically, I am excited by a novel technique first described by Yamane at the 2016 ASCRS meeting in New Orleans,1 the double-needle technique for flanged intrascleral posterior chamber IOL (PCIOL) fixation. After performing this method of IOL implantation in several eyes with inadequate capsular support, I developed a modification, which I describe in detail herein.
A BRIEF HISTORY
Historically, polymethyl methacrylate (PMMA) IOLs with holes were used for PCIOL placement, but a large corneoscleral incision was required to accommodate the rigid lens material. Such large incisions increase the risk for intraoperative hypotony and suprachoroidal hemorrhage and can increase the potential for postoperative astigmatism. Suturing these lenses to the sclera was cumbersome, because it required the surgeon to keep the strands of suture straight. Furthermore, the polypropylene suture material (Prolene; Ethicon) had the potential to degrade over time, resulting in late IOL dislocation.2 In the absence of flaps, scleral suture fixation could lead to lifelong risk of suture infection and a nidus for endophthalmitis. Iris fixation of PCIOLs has also been proposed, but it can lead to complications that include pupillary distortion, pigment dispersion, dislocation, or uveitis-glaucoma-hyphema (UGH) syndrome.3
The Artisan iris-claw IOL (Ophtec) is another modality for secondary IOL fixation. The IOL’s enclavation claws hold the IOL to the iris, and fixation can be performed on either the anterior or posterior iris. A large corneoscleral incision is required due to the PMMA material, and as stated, this comes with risks. As with any iris-fixation technique, complications can include chronic inflammation, cystoid macular edema, and iridodonesis.4 Dislocation of an anteriorly placed IOL could lead to corneal endothelial cell loss, while dislocation of a posterior-placed IOL would fall into the vitreous space.5
Several developments have helped to improve results with scleral-fixation techniques for PCIOLs. The off-label use of Gore-Tex CV-8 as a 7-0 suture (W.L. Gore & Associates) is an improvement over Prolene because it does not break or degrade, and it has improved the safety and long-term success of lens implantation.6 The development of intraocular instruments has also helped overcome technical challenges in PCIOL placement, but there is still the need for surgical gymnastics to pass, loop, and tie all the suture strands.
The enVista MX60 (Bausch + Lomb) is a hydrophobic IOL with holes that can be looped with polypropylene for suture fixation;7 however, Gore-Tex suture is probably the better material for long-term stability. Although this is an acceptable option, care must be taken to place the sutures in the proper position and to balance the suture tension; if not achieved, IOL tilt or malposition can result.
Even when perfectly executed, all of the techniques thus far described are susceptible to “IOL-donesis.” This is because there is only single-point fixation on either side, which can lead to micromovements of the IOL and, ultimately, affect lens stability and quality of vision. The Akreos AO60 lens (Bausch + Lomb), owing to its shape and configuration, provides excellent four-point fixation and reduces IOL-donesis,8 but it requires Gore-Tex sutures, which again is a cumbersome technique. Moreover, the hydrophilic lens material is less than ideal for eyes that could require future retinal surgery or endothelial keratoplasty with intraocular gas, potentially causing IOL opacification.
Dr. Kim demonstrates his modifications to and pearls for the Yamane double-needle technique.
Intrascleral haptic fixation with the glued IOL technique has gained popularity, as it does not involve suturing and provides stable IOL fixation through scleral tunnels; however, it requires extensive conjunctival and scleral flap dissection and intraocular forceps to pull the haptics through sclerotomies. Despite seemingly stable scleral fixation, there have been case reports of haptic extrusion and late IOL dislocation.9
All of the proposed techniques for PCIOL fixation have one characteristic in common: they are time consuming and require a lot of effort from the surgeon. Anterior chamber (AC) IOLs are a technically easier and more conservative approach. Although they can work well, ill-positioned AC IOLs can cause UGH syndrome, corneal decompensation, or chronic pain syndrome. Contact lenses are the least invasive nonsurgical option, but aphakia is not a great refractive option given the patient’s choices.
A NOVEL APPROACH
In flanged intrascleral PCIOL fixation with the double-needle technique, the leading haptic and optic are delivered into the AC through a keratome incision. Ninety degrees away, a 30-gauge, thin-walled needle is used to create a sclerotomy incision posterior to the limbus and pierced into the vitreous space. Intraocular forceps are used to thread the leading haptic of the IOL into the lumen of the needle. Using another 30-gauge needle, the surgeon creates a second sclerotomy incision 180º away and threads the trailing haptic into the lumen of the needle with intraocular forceps in similar fashion. Both needles are externalized from each sclerotomy, and the surgeon uses a handheld cautery to create a flange or bulb at the tip of the haptics to secure the IOL. He or she then pushes the haptics into the eye and the flanges flush with the sclera and then reapproximates the conjunctiva.
Yamane’s double-needle technique is an elegant approach to IOL fixation, because it bypasses all the surgical gymnastics required in the aforementioned techniques, and according to study results, is a simple and minimally invasive method for achieving good IOL and haptic fixation (see A Closer Look).10 Even though the procedure looks deceptively easy, the double-needle technique is not without challenges. The type of three-piece IOL can either complicate or enhance the surgeon’s ability to perform the technique.
Most three-piece PCIOLs have thin haptics that are prone to bending and breaking with manipulation. The double-needle technique requires an IOL that can withstand significant manipulation. I prefer the Aaris EC-3 PAL IOL (Aaren Scientific). There may be other viable alternatives internationally, but in the United States, I believe this is the best choice. The durable haptics of the EC-3 PAL, made of resilient polyvinylidene fluoride, resist bending or breaking and make the IOL well suited for the double-needle technique. Internalizing the haptics into the lumen of the needles is the greatest challenge with this technique. Risks include iris trauma, corneal trauma, slippage of the haptics into the vitreous space, and tilt or decentration of the IOL, all of which can result in prolonged surgical time and manipulation.
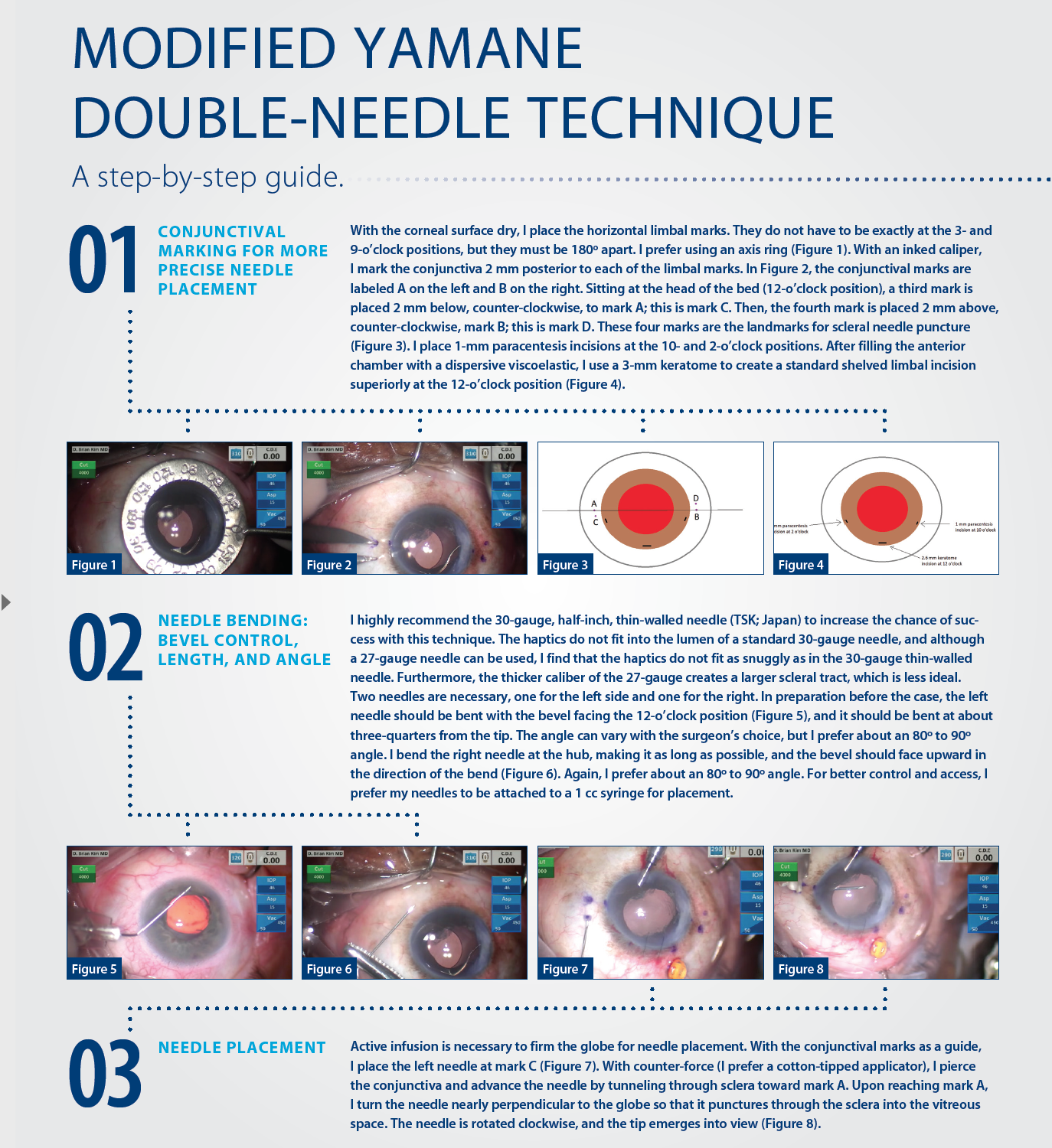
A MODIFICATION
My modification to the double-needle technique, which I believe minimizes the challenges associated with Yamane’s original technique, is outlined in the accompanying sidebar on pages 30 and 32. There are three “housekeeping” points with the use of my technique:
No. 1. A soft globe is difficult to pierce with a needle, so infusion is essential.
An AC maintainer or pars plana infusion can be used, depending on the surgeon’s experience and preference. I prefer a 23-gauge Lewicky AC maintainer (Katena), because it fits through a 1-mm paracentesis and seals easily, and I place the AC maintainer away from where most of the surgical manipulation will occur. I prefer an inferior placement when working at the head of the bed.
No. 2. Every patient should have a peripheral iridectomy, either laser or surgical, to minimize the risk of pupillary block.
All capsule-less IOL fixation techniques are prone to pupillary block glaucoma. I prefer a surgical peripheral iridectomy with the vitrector through a limbal incision. I have a separate low-flow irrigation-aspiration-cut setting (aspiration flow rate 12 with linear control, vacuum 350 with linear control, and 30 cut per minute rate) for this on my phaco machine (Centurion Vision System; Alcon). From a limbal incision, I place the port down onto the peripheral iris; pressing the footpedal will initiate aspiration. As the vacuum builds, occlusion will occur. Further depression initiates the cutter, and a round peripheral hole will be created.
No. 3. Every IOL exchange or secondary IOL placement in the absence of capsular support should be preceded by a limited anterior vitrectomy.
This can be achieved either through an anterior or pars plana approach to ensure that the dislocated IOL and AC are free of vitreous. I prefer to use a 23-gauge 4-mm valved trocar (Alcon) for pars plana vitrectomy, placed approximately 3 mm posterior to the limbus. It is important to do adequate vitrectomy to ensure there is no vitreous in the AC and anywhere around the offending IOL before explantation.
A CLOSER LOOK
Review of the clinical outcomes of Yamane’s transconjunctival intrascleral IOL fixation technique
By Laura Straub, Editor-in-Chief
In Yamane’s original technique of transconjunctival intrascleral IOL fixation, a pair of 30-gauge thin-walled needles are used to create two angled incisions parallel to the limbus and to externalize and cauterize the IOL’s haptics. The resultant flange is then pushed back and fixed into the scleral tunnels to achieve haptic fixation.
According to a recent study published in Ophthalmology,1 this flanged IOL fixation technique can be used to achieve good IOL fixation and firm haptic fixation in eyes with inadequate capsular support. A total of 100 eyes from 97 consecutive patients were enrolled in the prospective, noncomparative, interventional case series. In all cases, patients underwent posterior chamber sutureless IOL implantation due to aphakia, a dislocated IOL, or a subluxated crystalline lens.
Postoperatively, exact IOL centration and axial stability were achieved. Further, the mean BCVA improved from 0.25 logMAR preoperatively to 0.11, 0.09, 0.12, and 0.04 logMAR at 6, 12, 24, and 36 months, respectively (P < .01, P < .01, P = .03, and P = .10, respectively). The researchers also reported a decrease in the mean endothelial cell density from 2,341 cells/mm2 preoperatively to 2,313, 2,240, 2,189, and 2,244 cells/mm2 at 6, 12, 24, and 36 months, respectively (P < .01, P < .01, P < .01, and P = .17, respectively). There were no significant surgical complications such as postoperative retinal detachment, endophthalmitis, or IOL dislocation; however, IOL capture occurred in eight eyes, vitreous hemorrhage in five, and cystoid macular edema in one.
1. Yamane S, Sato S, Maruyama-Inoue M, Kadonosono K. Flanged intrascleral intraocular lens fixation with double-needle technique. Ophthalmology. 2017;124(8):1136-1142.
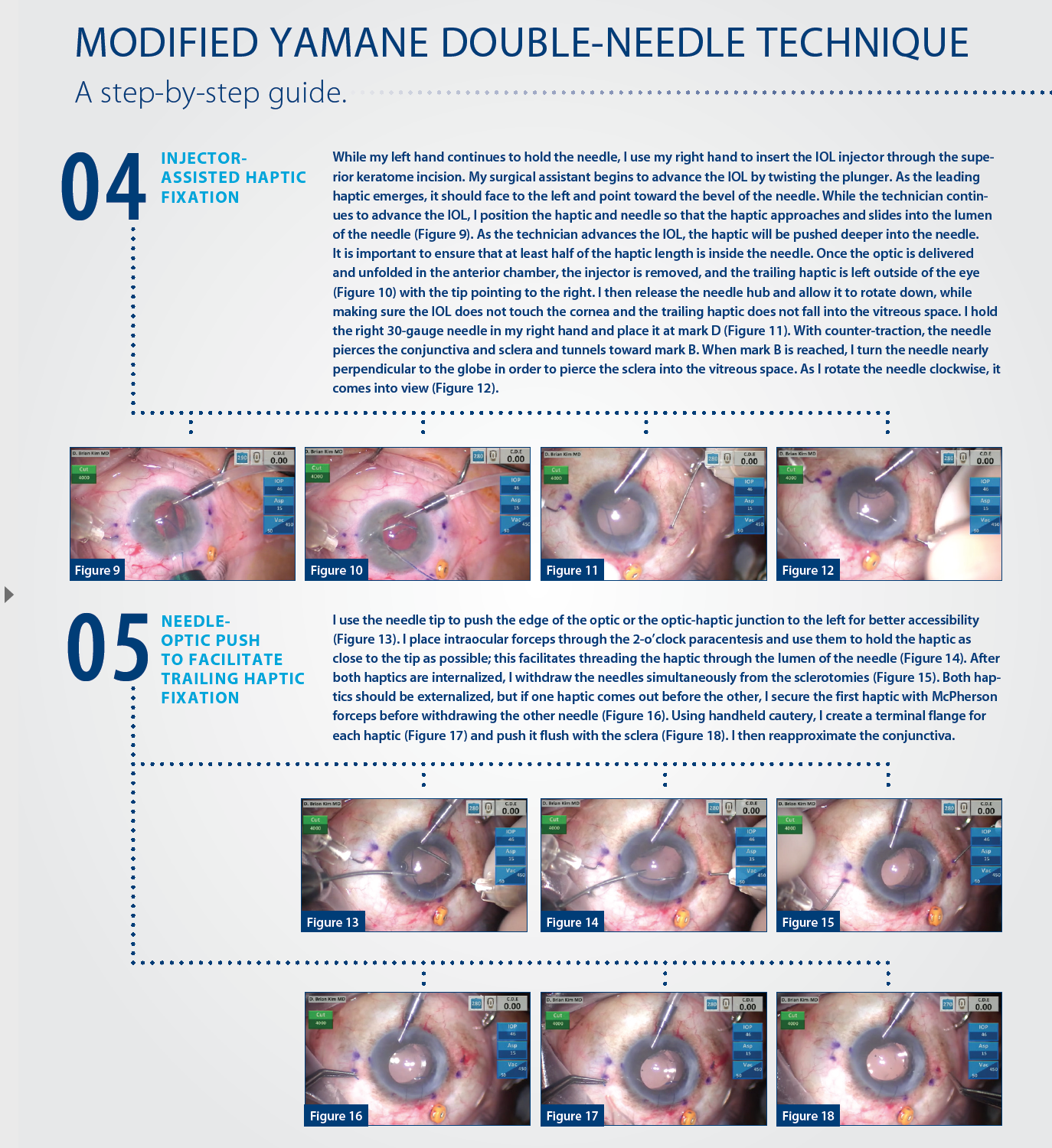
OTHER TIPS
When attempting IOL implantation with my modified technique, I have found help in the following pointers.
No. 1. Conjunctival marking helps.
Poor haptic placement can lead to IOL tilt or decentration. Too far posterior and the needle may puncture the retina; too far anterior and the IOL can cause pigment dispersion or UGH syndrome. Conjunctival marking reduces these risks and promotes more precise haptic placement.
No. 2. Precise bevel position, needle length, and needle bend help the surgeon to insert the haptics into the needles more easily and safely.
The bevel should always face the direction of the haptic approaching the needle. With this accomplished, the bevel more easily engages the haptic and facilitates its passage through the needle. On the other hand, improper bevel position can make insertion of the haptic into the needle more difficult. Too short a needle from bending too close to the tip will make reaching the haptic for insertion difficult. Bending the needle is also important for safety. If the left needle is not bent, when released, too much of the needle can slide into the eye uncontrollably and potentially harm posterior segment structures.
No. 3. Injector-assisted haptic fixation bypasses several of the manual steps of the traditional technique.
Holding the injector is easy and familiar to the cataract surgeon and is a much more controlled approach than the one originally described by Yamane. By using the injector, the surgeon does not need to advance the lens and can focus on positioning the leading haptic to facilitate its entrance into the needle as the surgical technician advances the IOL. The force created from advancement of the injector pushes more of the haptic into the needle without any surgeon intervention. If concerned, he or she can place an intraocular forceps through the 10-o’clock paracentesis to push the haptic into the needle lumen even farther; however, in my experience this has not been necessary.
No. 4. The needle-optic push can be used to grasp the haptic of an IOL.
The polyvinylidene haptics of the EC-3 PAL lens are thick and rigid, and they resist deformation. These characteristics makes the IOL ideal for long-term stable scleral fixation, but they also pose a challenge when I am trying to fixate the trailing haptic. Needle-optic push is necessary to expose the distal haptic to the intraocular forceps. Without this maneuver, the forceps can only reach the proximal haptic; thus, it is more difficult to control the haptic when attempting to thread the needle. Needle-optic push places significant force on the leading haptic and optic; surgeons might fear that the maneuver could cause the haptic to dislodge from the needle and into the vitreous space, but this has not been my experience.
I theorize that the haptic is stable for three reasons. First, there is a snug fit between the haptic and needle lumen, which resists slippage. Second, since the needle is straight and the haptic is curved, the haptic applies frictional forces on the inside of the needle, which resists slippage. Third, in order for the haptic to come out, it would have to experience significant torsional force in the clockwise direction, which it does not with this maneuver.
Once I became comfortable and confident with the needle-optic push, the distal haptic was much easier to grab with the intraocular forcep, resulting in a smoother needle insertion.
No. 5. When both needles are withdrawn, care must be taken to pull them out in the direction of the needle tracts.
Both haptics may not release simultaneously, thus I do not prefer a simultaneous pull-out as originally described. If one haptic comes out first, the surgeon should secure it with McPherson forceps before attempting to externalize the second to prevent the haptic from slipping back into the eye. Cautery should be used sparingly to form a small flange on each haptic. Too much cautery will shorten the haptic and increase the risk of asymmetric haptic length and the potential for IOL decentration. The flange must be pushed into the eye so that it is flush with the sclera, and the conjunctiva must be reapproximated.
Postoperatively, the haptics are barely visible under the conjunctiva. I have not experienced conjunctival erosions, infections, or other complications from this technique. There is no IOL-donesis, and the lenses are well centered.
CONCLUSION
I have tried many different IOL techniques in the absence of capsular support, and I am grateful to the innovators who have developed them. I feel Yamane’s double-needle technique is a welcome improvement, and it introduces a radically different way of thinking about these cases. I hope the modifications to the double-needle technique that I suggest here will encourage surgeons to try it and will enhance their consistency, efficiency, and safety.
1. Yamane S. Transconjunctival intrascleral IOL fixation with double-needle technique. Film presented at: The ASCRS Symposium on Cataract, IOL and Refractive Surgery; May 6-10, 2016; New Orleans, LA.
2. Price MO, Price FW Jr, Werner L, Berlie C, Mamalis N. Late dislocation of scleral-sutured posterior chamber intraocular lenses. J Cataract Refract Surg. 2005;31:1320-1326.
3. Kaiura TL, Seedor JA, Koplin RS, et al. Complications arising from iris-fixated posterior chamber intraocular lenses. J Cataract Refract Surg. 2005;31:2420-2422.
4. Forlini M, Soliman W, Bratu A, Rossini P, Cavallini GM, Forlini C. Long-term follow-up of retropupillary iris-claw intraocular lens implantation: a retrospective analysis. BMC Ophthalmol. 2015;15:143.
5. Anbari A, Lake DB. Posteriorly enclavated iris claw intraocular lens for aphakia: long-term corneal endothelial safety study. Eur J Ophthalmol. 2015;25:3:208-213.
6. Khan A, Gupta O, Smith R, et al. Scleral fixation of intraocular lenses using Gore-Tex suture: clinical outcomes and safety profile. Br J Ophthalmol. 2016;100(5):638-643.
7. Rho S, Song WK, Sung Y, et al. Scleral fixation technique using a hydrophobic foldable intraocular lens with ring-shaped connecting bridges. J Cataract Refract Surg. 2015;41(2):262-267.
8. Terveen D, Fram N, Ayres B, Berdahl, J. Small-incision 4-point scleral suture fixation of a foldable hydrophilic acrylic intraocular lens in the absence of capsule support. J Cataract Refract Surg. 2016;42(2): 211-216.
9. Kumar DA, Agarwal A, Packiyalakshmi S, Jacob S, Agarwal A. Complications and visual outcomes after glued foldable intraocular lens implantation in eyes with inadequate capsules. J Cataract Refract Surg. 2013;39:1211-1218.
10. Yamane S, Sato S, Maruyama-Inoue M, Kadonosono K. Flanged intrascleral intraocular lens fixation with double-needle technique. Ophthalmology. 2017;124(8):1136-1142.




