
Dry eye disease (DED) can affect ocular surgery. One of the more obvious ways is that undetected disease may influence how successful patients consider their procedure to have been. If they do not learn that they have DED before the postoperative period, they may blame the procedure. Fair or not (and in most cases, there is not much correlation), DED discovered after surgery is viewed as a complication, whereas patients who receive forewarning can at least understand the nature of any potential visual symptoms.
In addition, undetected DED is a leading cause of refractive surprises after cataract or refractive surgery. The condition can cause visual disturbances on its own, which is one potential explanation of why the refractive target was not met. A potentially more serious cause of postoperative refractive surprise is that DED may affect the accuracy of preoperative measurements (biometry, topography, and keratometry). Inaccurate biometry can lead to inappropriate IOL power selection for cataract patients. For those with astigmatism, the wrong toric IOL power or axis of alignment can cause significant problems. In the setting of refractive surgery, the ablation pattern may be off center or inaccurate if DED affected the topography readings, and as topography-guided LASIK becomes a more popular option, undetected DED becomes a greater cause for concern, because it leads to corrupted data and therefore corrupted ablation profiles.
AN IRREGULAR TEAR FILM AND CATARACT SURGERY
DED can either be aqueous deficient or evaporative in nature, and both types may be present simultaneously. While the etiologies are distinct, irregular tear osmolarity is a feature of both subtypes. On tear osmolarity testing (TearLab), a score of 308 mOsm/L or higher is well correlated to an abnormal tear film, and a difference in the scores greater than 8 mOsm/L is indicative of tear film instability.1
A score of 308 mOsm/L or higher on osmolarity testing is suggestive of DED but is not necessarily diagnostic on its own. The presence of disease must still be validated through clinical evaluation and an additional workup, including vital dye staining, confirmatory testing, and the use of other diagnostic modalities as appropriate and as dictated. Perhaps more important is how tear osmolarity scores change over time. DED is a condition characterized by tear film instability, so it seems intuitive that fluctuating osmolarity levels would correlate with irregularities at the tear film level.
In the context of surgical patients, an abnormal tear film (osmolarity ≥ 308 mOsm/L) indicates a risk of inaccurate keratometry, anterior corneal astigmatism assessment, and IOL power calculations. Using a threshold of 316 mOsm/L, Epitropoulos et al showed that IOL power calculations could vary as much as 0.50 D in patients with hyperosmolarity who were measured at different visits.2 Of note, there were no significant differences in IOL power calculations at different clinic visits among eyes with osmolarity scores considered “normal.” I have observed this phenomenon in my practice, where the same patient with DED had different biometric measurements during separate examinations, resulting in vastly different suggested IOL powers (Figure).
Inaccurate IOL power calculations have potential consequences for any patient undergoing cataract surgery. The issue may be magnified in surgical candidates considering premium lenses, who tend to have elevated expectations. At a clinical level, an incorrectly powered presbyopia-correcting IOL may necessitate an additional procedure such as LASIK, piggyback IOL, or lens exchange.
For the patient receiving a toric implant, an incorrect power may be disastrous, because the new lens may magnify the preexisting refractive error. Moreover, DED is a condition affecting the tear film at the outermost layer of the cornea—precisely where topography and keratometry are scanning to map the shape and potential irregularities of the cornea. It makes sense, then, that an irregular tear film has the potential to cause a miscalculation of both the magnitude and axis of astigmatism.
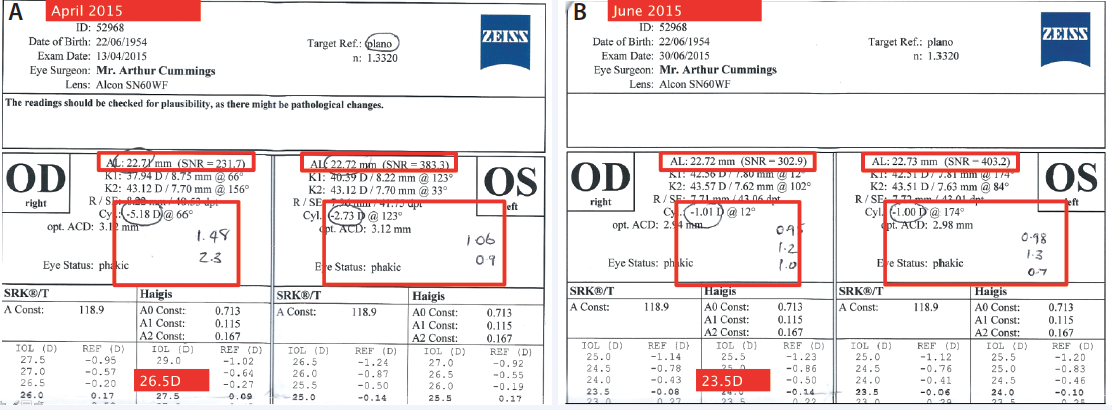
Figure. Initial consultation with a patient who had DED and was being evaluated for a cataract. Preoperative measurements suggested an axial length of 22.72 mm OD and cylinder of -5.18 D at 66°, which would necessitate an IOL power of 26.50 D for a plano refractive target (A). During a subsequent evaluation after successful therapy for DED, axial length measured 22.71 mm OD with cylinder of -1.01 D at 12°, which would suggest an IOL of 23.50 D for a plano refractive target (B ). Additionally, the magnitude of astigmatism varied greatly in the dry eyes and was far more consistent and in agreement once the DED was treated. The other numbers written on this IOLMaster printout (Carl Zeiss Meditec) are the cylinder values from the Pentacam (Oculus Surgical) and the Lenstar (Haag-Streit).
INSTITUTIONAL STUDY: DRY EYE AND REFRACTIVE SURGERY
Because irregularities at the level of the tear film appear to affect the accuracy of preoperative readings, I became curious as to the extent to which they could impede the laser’s programming ability to design an accurate ablation pattern for patients considering topography-guided LASIK. Available to surgeons in Europe and approved in the United States for patients undergoing a primary LASIK procedure, this form of laser vision correction uses topography to create individualized treatment plans that eliminate the effect of aberrations at the corneal surface. The ablation pattern for topography-guided LASIK is constructed using a Placido disc topographer with data gathered via reflection of the tear layer.
Similar to the rationale of the study by Epitropoulos et al, my colleagues and I sought to determine if tear osmolarity could serve as an index for the accuracy of the topographic map used for ablation in eyes undergoing topography-guided LASIK. For our 2015 study, we performed osmolarity testing on 20 patients and then evaluated if the topographic map generated by the WaveLight Allegro Topolyzer Vario (Alcon) was accepted.
Our study demonstrated that the higher the osmolarity was, the more likely the software was to reject the topographic data due to poor-quality acquisition. On the other hand, the treatment plan was more likely to be accepted in eyes with a normal osmolarity score.
Based on this study, osmolarity appears to be a useful surrogate for the accuracy of preoperative data. When the topographic map is automatically approved, it means less need to manually override or correct the treatment plan and a higher potential to perform an accurate ablation.
CONCLUSION
Differing measurements on the various technologies used for preoperative planning can cause confusion and complicate IOL power selection for cataract patients or planning an ablation pattern for refractive surgery. Tear osmolarity testing may be useful for detecting abnormality common in both aqueous-deficient and evaporative DED. It may also serve as an index of the accuracy of preoperative assessments, which should provide surgeons with more confidence in their surgical plan as well as reduce the potential for postoperative refractive surprises.
My colleagues and I look forward to validating our initial findings in an institutional study that demonstrated the potential application of tear osmolarity testing for predicting the accuracy of topographic maps used for constructing LASIK ablation patterns. It is perhaps too early to suggest changes to clinical protocols based on our results, but they provide an intriguing additional application of a validated diagnostic used for assessing DED.
1. Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151:792-798.
2. Epitropoulos AT, Matossian C, Berdy GJ. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41(8):1672-1677.
Arthur B. Cummings, MB ChB, FCS(SA), MMed(Ophth), FRCS(Edin)
• consultant ophthalmologist, Wellington Eye Clinic and Beacon Hospital, Dublin, Ireland
• abc@wellingtoneyeclinic.com
• financial disclosure: consultant to Alcon, TearLab, and WaveLight


