Medical practices have endured a lot of change in the past year with the constant threat of COVID-19. From the lockdown phase to the reopen phase to the recover and protect phase, one thing remains constant: A more robust process for safe patient care is crucial to keeping office doors open.
THE BENEFITS OF GENETIC TESTING
With its Active Coronavirus Testing & Safety (ACTS) program and Corporate Testing Solution, Avellino is committed to helping surgical and procedure-oriented practices enhance their patient and staff safety protocols. Avellino is a precision medicine company that creates new opportunities for earlier disease protection through genetic testing. The company has leveraged the technology of its proprietary DNA diagnostic Universal test (for the detection of genetic indicators positively associated with corneal diseases) to create its Avellino SARS-CoV-2/COVID-19 genetic test, AvellinoCoV2. Avellino obtained Emergency Use Authorization for the test in March 2020—one of the first independent labs in the United States to do so. Avellino has since performed more than 1 million AvellinoCoV2 tests.
AvellinoCoV2 is a noninvasive swab or saliva test used to detect the SARS-CoV-2 virus and assist in the diagnosis of COVID-19 infections. A positive result with this test generally indicates an active infection; however, the test should not be used as the sole basis for treatment or other patient management decisions. For the safest results and a proper medical diagnosis, testing must be combined with clinical observations, patient history, and epidemiological information.
THE ACTS PROGRAM
The ACTS program provides practices with a guaranteed supply of AvellinoCoV2 genetic tests, which can be used to help practices detect active carriers of the SARS-CoV-2 virus with or without symptoms. The ACTS program also includes the option for a multiplex respiratory panel (AvellinoCoV2-Respiratory) that includes influenza A/B/C and severe acute respiratory syndrome-like viruses. Testing for respiratory syncytial virus (RSV) will be an addition to that panel in the near future.
It is recommended that the AvellinoCoV2 test be used as a screening tool in asymptomatic patients and that the AvellinoCoV2-Respiratory test be used for patients with symptoms associated with coronavirus. Used together, the AvellinoCoV2 and AvellinoCoV2-Respiratory tests can provide a differential diagnosis, allowing the practice’s personnel to properly quarantine people—both staff and patients—and sanitize the practice. Inclusion of the RSV test is also recommended in children. Because they often now have caretakers who are grandparents, it’s critical to be able to differentiate between COVID-19, the flu, and RSV in the youngest patient population.
The Families First Coronavirus Response Act, passed by the US government in March 2020, mandates that testing for COVID-19 is covered by insurance if an individual shows signs or symptoms compatible with COVID-19, as well as if an asymptomatic individual has had known or suspected recent exposure to SARS-CoV-2. This act also prohibits bounce billing, or charging patients more than the agreed upon rates. AvellinoCoV2 is covered by the following providers: Aetna, Anthem Blue Cross, Blue California, Cigna, Coventry Health Care, Health Alliance, HealthLink, Humana, Illinicare Health, Kaiser Permanente, Medicaid, Medicare, Meridian, Molina Healthcare, NALC Health Benefit Plan, Sutter Health, Tricare, TriWest Healthcare Alliance, and United Healthcare. Avellino continues to work with federal and local governments and private partners to increase access to its real-time polymerase chain reaction (RT-PCR) testing. Outside of insurance coverage, the cost of a single test is $200 for the AvellinoCoV2 test and $300 for the AvellinoCoV2-Respiratory test.
THE BASICS
It is important to know the basics about testing for SARS-CoV-2, including the types of testing, sample collection methods, testing results, and turnaround times.
Types of testing. Diagnostic testing determines the presence of an active SARS-CoV-2 infection, and antibody testing looks for the presence of coronavirus antibodies within the blood. Antibodies may take up to weeks to develop after exposure to a virus and may stay in the blood for several weeks after recovery. Therefore, antibody testing should not be used to diagnose COVID-19 or to determine whether or not someone may be infectious to others.
The two types of diagnostic tests available to detect viruses are molecular and antigen. AvellinoCoV2 is a RT-PCR molecular test that detects the presence of viral RNA in the body. RT-PCR testing is the gold standard in diagnostic testing and the most accurate form of SARS-CoV-2 virus detection.
Sample collection methods. Three types of collection can be used with the AvellinoCoV2 test—an oropharyngeal swab, a nasopharyngeal swab, and saliva collection. The sample, 1 mL in volume, is placed in an iSWAB microbiome collection tube. Some medical practices require nasal swabs over saliva collection for adult patients because the nasal cavity carries a higher percentage of viral load. However, saliva collection is a great option for younger patients and children.
Test results. Results of diagnostic tests can be positive, negative, or inconclusive (Figure 1). Positive and negative test results indicate that an individual is positive or negative at that moment. An inconclusive result requires recollection of the sample for whatever reason, including because of poor sample quality or inadequate sample volume.
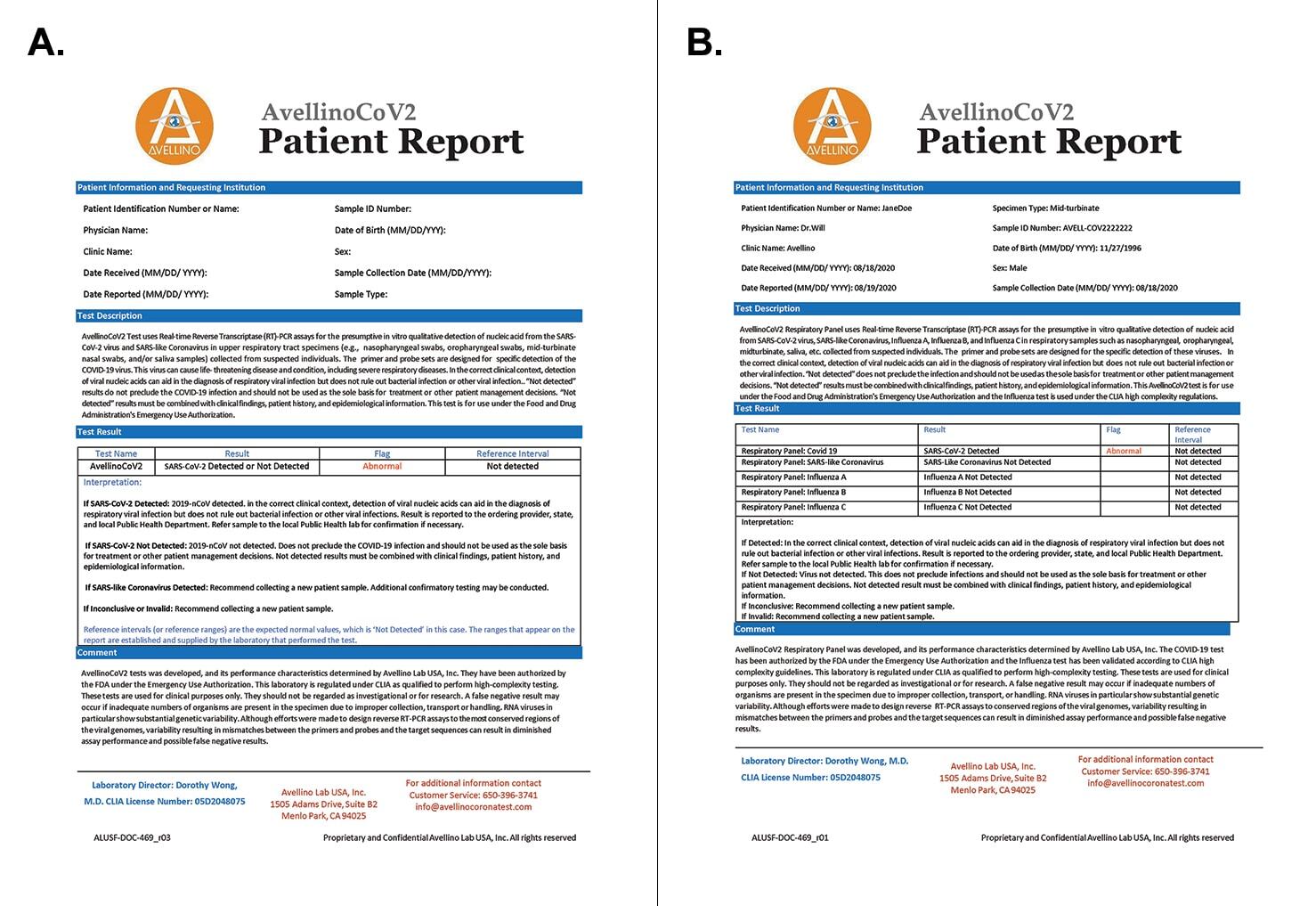
Figure 1. Sample patient reports for the AvellinoCoV2 (A) and AvellinoCoV2-Respiratory (B) genetic tests.
Turnaround times. The turnaround time (TAT) is defined as the usual number of days from the date that the swab specimen arrives in the testing facility to when the result is released to the ordering health care provider via a patient report. With the ACTS program, most results are typically returned within 24 to 48 hours of receiving the sample. However, TAT can vary due to sudden surges in testing volumes around the country. Please check with your Avellino representative on current TAT. All positive and inconclusive test results are rechecked to ensure accuracy.
SAFETY PROTOCOLS
A number of different protocols can be used to introduce SARS-CoV-2 virus testing into a medical practice. Regardless of the chosen protocol, following that protocol accurately is key to safe and reliable outcomes. The Avellino ACTS program staff and patient COVID-19 testing process is outlined in Figure 2. These current coronavirus protection measures include:
- Regular testing of staff to protect employee and patient health;
- Testing of all surgical and procedure patients to avoid the risks associated with operating on carriers of COVID-19;
- Pre-procedure quarantine for patients;
- Use of gloves and face coverings; and
- Continuous sterilization in all areas of the medical practice.
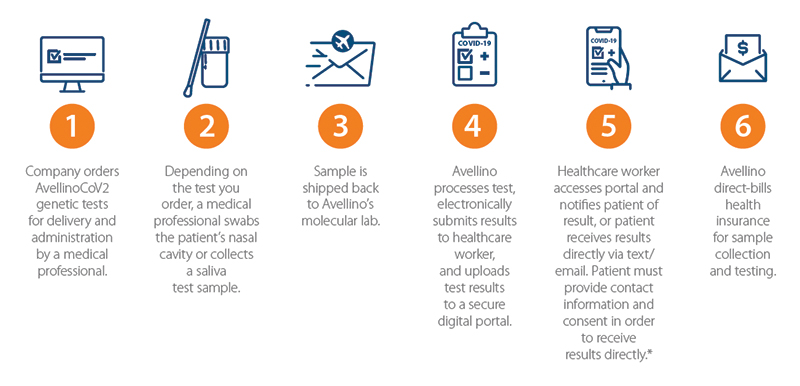
Figure 2. Avellino ACTS program staff and patient COVID-19 testing process.
CONCLUSION
Following a strict protocol like that established in the ACTS program by Avellino allows practices to stay open safely. Helping to identify individuals with the active virus and mandating a quarantine period ensures that everyone else in the practice—staff and patients—are protected as much as they can be during this pandemic.
For more information on the ACTS program and the AvellinoCoV2 genetic test, visit www.Avellino.com or email info@avellino.com.




