Higher levels of irrigation pressure were historically necessary to allow for adequate chamber stability with efficient aspiration flow rates and vacuum. These pressures helped to compensate for dynamic pressure losses and shallowing of the anterior chamber resulting from flow. High irrigation pressures, on the other hand, produce intraoperative IOPs that are many times higher than the physiologic IOP, which have negative consequences for ocular tissues.
One approach, introduced in the late nineties, aimed to lessen the impact on ocular tissues by lowering the intraoperative IOP. The procedure was termed “slow-motion phaco” and specifically targeted compromised eyes.1,2 As the name suggests, lowering the IOP required a reduction of aspiration rates and vacuum, which resulted in significantly prolonged surgery time.3
Today’s advanced technology has overcome this challenge by moving away from gravity-based systems to Active FluidicsTM technology (Alcon), as well as integrating response systems that perform early detection and compensate for surge effects. These advances enable surgeons to operate at a near physiologic IOP with equivalent surgical efficiency while protecting ocular structures.
Surgical efficiency during cataract surgery
A past hurdle in adopting a lower IOP was often the assumption that the surgical efficiency may be compromised. Recent studies investigated the efficiency of cataract surgery at high and near physiologic IOPs and maintained almost identical surgical parameters (aspiration rates and vacuum). Overall, the use of CENTURION® with ACTIVE SENTRY® (Alcon) at lower and higher IOPs resulted in the same case time4,5, aspiration time4,5, and ultrasound time4,6 (Table 1). However, one retrospective study reported significantly improved case time and aspiration time with lower IOP settings.7 BSS fluid usage is also significantly reduced with lower IOP settings.4,5,7
Impact of IOP on the anterior chamber stability
It is crucial during cataract surgery to maintain a stable anterior chamber and provide sufficient space to operate. Surgery that would preserve the normal anatomy of the eye would be ideal, as it reduces the stress to the ocular structures. Beres et al7 suggested that high IOP during cataract surgery artificially distorts the anterior chamber, thereby negatively affecting ocular tissues. Conversely, it has been speculated that operating at lower IOP conditions may lead to an unstable chamber and potential collapse with surge events. Such conclusions may no longer hold validity in an era of enhanced surgical techniques, improved wound construction, smaller incisions, and technological advancements, including ACTIVE FLUIDICSTM and ACTIVE SENTRY®.
A pig eye study showcased the advancement of today’s technology and measured anterior chamber fluctuations under lower IOP conditions (30 mmHg) during phacoemulsification and occlusion break surges. The investigation confirmed that the anterior chamber remained stable.8 Surge duration and volume were significantly lower with CENTURION® ACTIVE SENTRY® as compared to CENTURION® with Ozil8 (Alcon). Contrary, gravity-based systems collapsed the anterior chamber and did not enable measurements of surge duration and volume.
Reduced fluctuation of the anterior chamber and surge volume are directly related to the integrated pressure sensor in the ACTIVE SENTRY® handpiece and QuickValve™ technology. The sensor rapidly detects the onset of post-occlusion surge, and upon detection, responds by opening the fast-acting QuickValve™ within the cassette to supply BSS to the aspiration line. This allows CENTURION® with ACTIVE SENTRY® to compensate faster for occlusion break surge as compared to CENTURION® with ACTIVE FLUIDICSTM only (Figure 1).
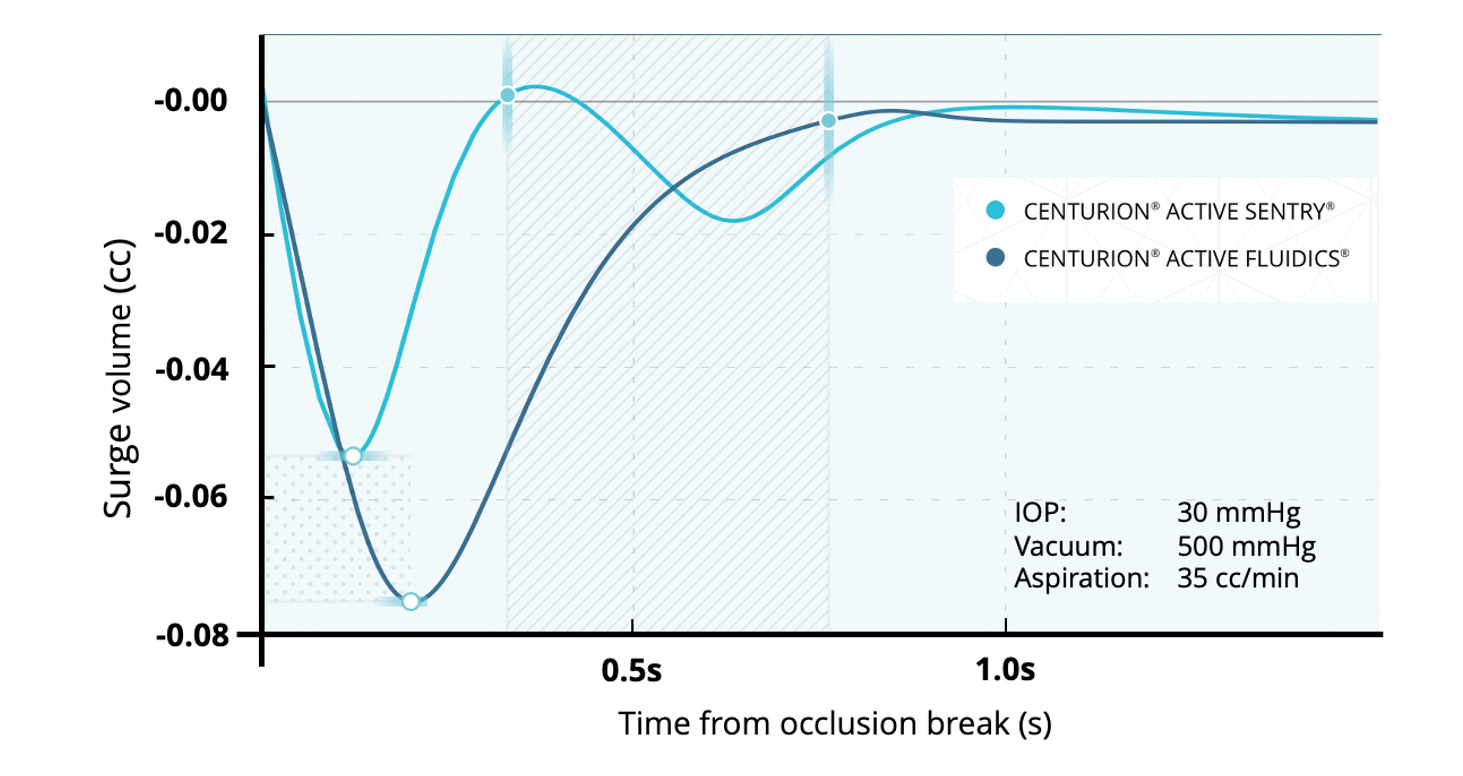
Figure 1. Occlusion break surge comparison9
Clinical Benefits for the Anterior Segment
More and more data are emerging on the ocular health benefits when operating at a near physiologic IOP versus high IOP conditions. Two recent contralateral eye studies compared high and near physiologic IOP (20 mmHg and 50 mmHg4, 28 mmHg and >61 mmHg5) using CENTURION® with ACTIVE SENTRY® with similar vacuum and aspiration rates.
Postoperative benefits of near physiologic IOP cataract surgery included:
- Less post-operative corneal swelling at up to 3 months4,5 (Figure 2)
- Better endothelial cell protection, as shown by higher endothelial cell density up to 3 months4,5 (Figure 3); smaller drop in endothelial cell density at 1 month5; higher percentage of eyes with hexagonal cells up to 3 months4; and no change in coefficient of variation, i.e. variation in endothelial cell size4
- Less anterior segment inflammation, as measured by cell5 and flare4,5 on day 1
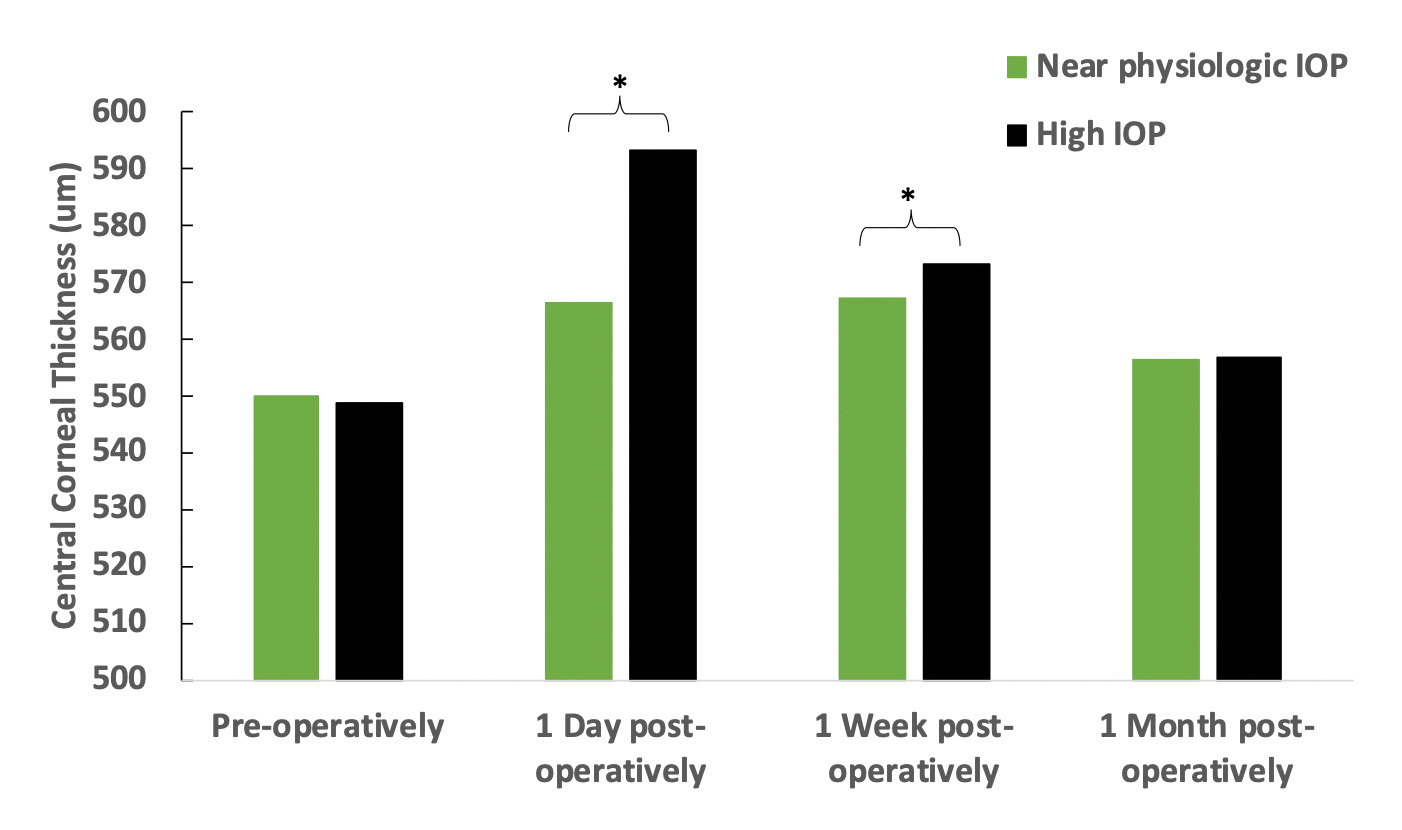
Figure 2. Central corneal thickness of eyes operated under high IOP (>61 mmHg, n = 27 eyes) and near physiologic IOP (28 mmHg, n = 27 eyes) conditions. The contralateral eye study showed that the corneal thickness was significantly less swollen in eyes operated at under lower IOP conditions on day 1 and 1 week postoperatively (*
5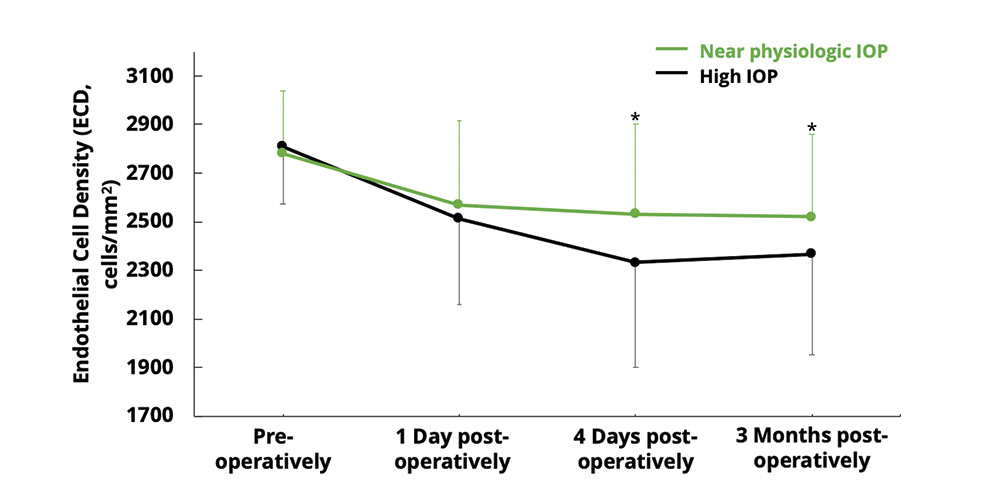
Figure 3. Endothelial cell density in eyes operated at under high IOP (50 mmHg, n = 58 eyes) and near physiologic IOP (20 mmHg, n = 57 eyes). ECD was significantly less impacted at day 4 and 3 months postoperatively under near physiologic IOP (*P < 0.01).4
Lower irrigation pressures may contribute to reducing the severity of a reverse pupillary block during cataract surgery. Reverse pupillary block or lens-iris diaphragm retropulsion syndrome (LIDRS) is known to have a higher likelihood to occur in vitrectomized eyes, longer axial lengths, and larger corneal incision sizes.10 Several reports associated the event of an active reverse pupillary block with significant discomfort for patients receiving phacoemulsification under topical or intracameral anesthesia; this was managed by lowering IOP.10-12
Clinical Benefits for the Posterior Segment
A few studies have started to investigate the impact of IOP on the posterior segment, but more needs to be done to evaluate not only short-term but also long-term effects.
Excessive flow to the Berger space (Figure 4) through weakened zonules and/or partially detached Wieger’s ligament may cause or extend ligament detachment during phacoemulsification and irrigation/aspiration.13 As a result, a complete separation of the anterior hyaloid membrane from the posterior lens capsule (ie, anterior vitreous detachment [AVD]) may occur.13 AVD occurrences during cataract surgery markedly increased from 0.9% preoperatively to 19.7% postoperatively,14 or 2.4% preoperatively to 56.1% postoperatively.15
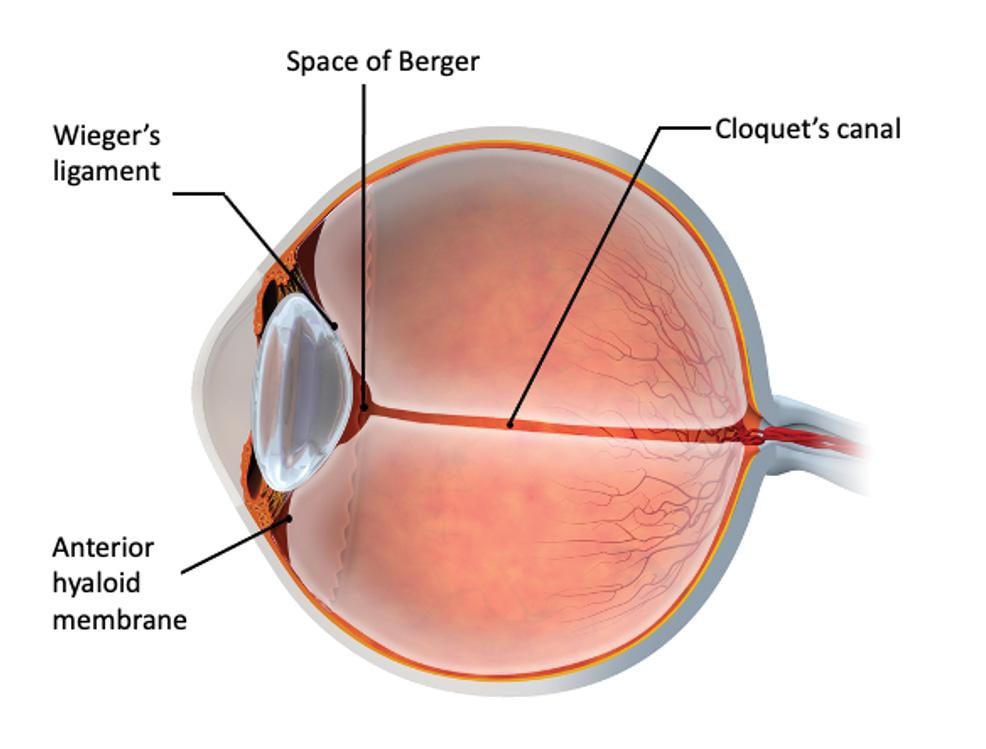
Figure 4. Depiction of the vitreolenticular interface along with the anterior hyaloid membrane, space of Berger and Wieger’s ligaments.
Evidence for an increased risk of anterior vitreous face (AVF) ruptures was provided by an experimental porcine eye model. Ultrasound-B scans visualized a forward and backward movement of the posterior capsule, as well as the Berger’s space during occlusions and occlusion breaks.12 Authors concluded that this trampolining movement contributed to AVF rupture in porcine eyes operated under high flow settings.16
Stresses to the anterior hyaloid membrane during cataract surgery were also associated with an increased postoperative presence of Berger’s space.13 Furthermore, higher IOP settings caused more anterior hyaloid membrane damage.6 While maintaining vacuum and aspiration rates the same, an IOP of 80 mmHg led to a significantly higher percentage of eyes with Berger’s space (42.5% of eyes) as compared to eyes receiving cataract surgery with an IOP of 30 mmHg (7.5% of eyes).6
With an extended disruption of the AVF and potential AVD, lens fragments, cellular material, or medication can migrate into the space between the posterior capsule and anterior cortical layers of the vitreous humor.13,15 Such a breach could potentially increase the risk of intraocular inflammation.15 Additionally, greater mobility of the anterior hyaloid membrane may also increase traction at the vitreous base, potentially leading to retinal detachment.13 Perhaps this is the reason why a significantly greater incidence rate of PVD was demonstrated in pseudophakic eyes (50.8%) as compared to phakic fellow eyes (20.8%).17
Changes in retinal blood flow as a result of an increased IOP may impact the macular vascular density after phacoemulsification, as well as potentially the recovery of visual acuity.18 Patients who underwent shorter irrigation times/ lower IOPs exhibited a smaller increase in macular thickness 1 week after surgery as compared to patients exposed to longer irrigation times/ higher stimulated dynamic IOP.19 Another study detected a lower macular vessel density in eyes with cataract surgery at an IOP of 40 mmHg versus surgery at an IOP of 70 mmHg.20 Also, cataract surgery at a high IOP (>65 mmHg) resulted in a reduction in FAZ, while lower IOP (28 mmHg) did not change FAZ in the first postoperative week.21
Impact on patient comfort
One very compelling argument for reducing intraoperative IOP during cataract surgery is patient comfort. An early case report of 136 patients undergoing cataract surgery using a gravity-based system cited that almost half of the patients experienced pain during irrigation steps of surgery.22 This pain was relieved by lowering the IOP, i.e. reduced aspiration rates or lower bottle height.22 In a more recent study comparing an IOP of 30 mmHg vs 80 mmHg, the patient’s subjective perception of pain as evaluated using the Wong Baker face scale was lower in patients that were operated under near physiologic IOP conditions.6 None of the eyes at near physiologic IOP had any pain, while 80% of eyes with higher IOP experienced sensations ranging from “hurt a little bit” to “hurts whole lot”.6
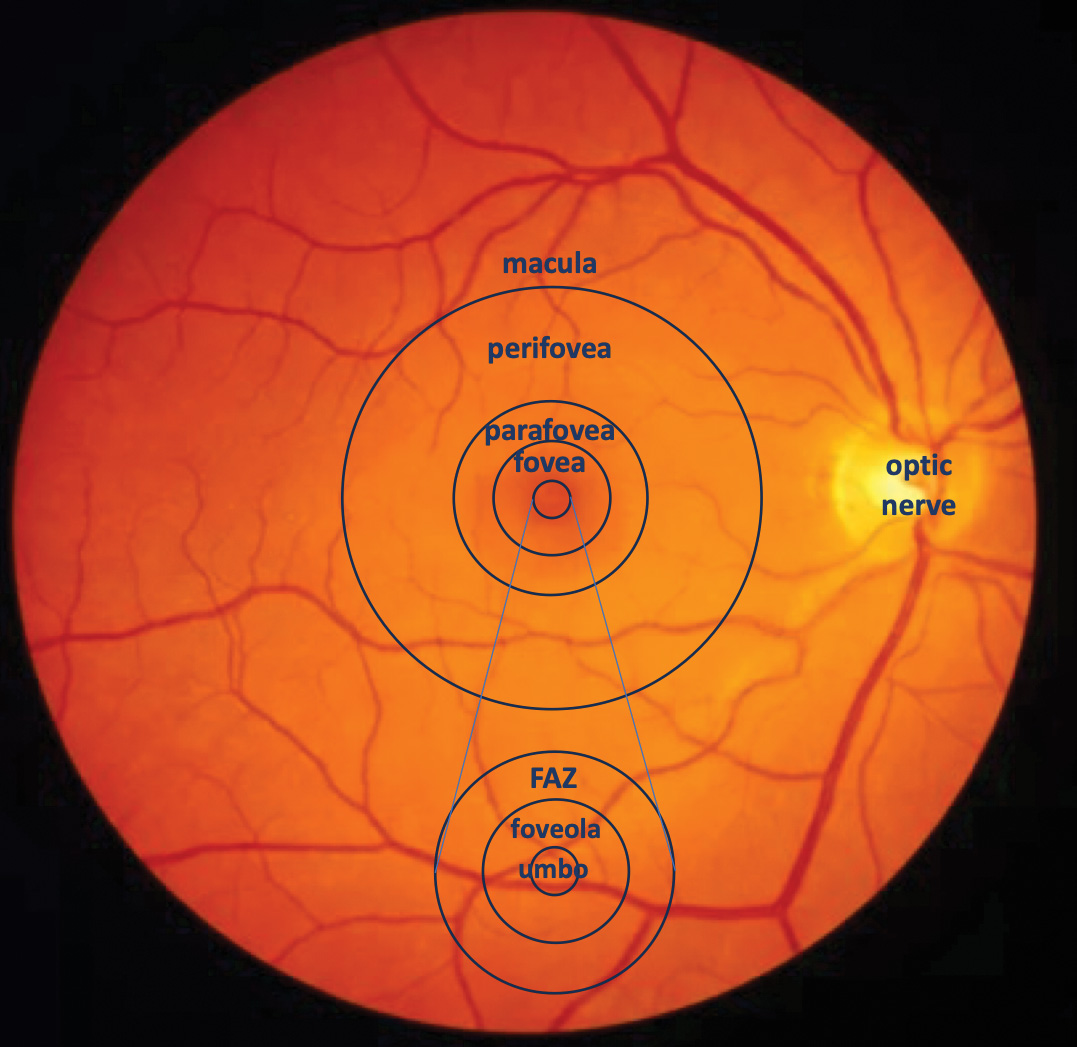
Figure 5. Depiction of the macular zones, including the foveal avascular zone (FAZ)
Conclusions
With technological and procedural advancements in ophthalmic surgery, it is possible today to operate at a near physiologic IOP and maintain or even improve surgical efficiency.4-7 A near physiologic IOP during cataract surgery causes less post-operative corneal swelling3-5,23,24, provides greater endothelial cell protection4,5,23 and triggers less anterior segment inflammation3-5,24 as compared to high IOP levels. Reverse pupillary block may also be better managed using lower intraoperative IOPs during surgery.10-12 Furthermore, stresses on the posterior capsule-anterior hyaloid membrane barrier may be reduced.6,16,25 While the impact on vision and visual recovery may not be fully clear yet, lower intraoperative IOP seemed to better protect the posterior segment of the eye than higher intraoperative IOP, with a lower increase in macular thickness18,19,26,27, less reduction of the FAZ area18,26,27 and lower increase in macular vessel density.20 Another argument for using lower IOP levels during cataract surgery includes the potential reduction in intraoperative pain.6 Taken together, CENTURION® with ACTIVE SENTRY® using a near physiologic IOP does not compromise surgical efficiency or anterior chamber stability and has less impact on the anterior and posterior structures of the patient’s eye.
© Alcon Inc. 1/24 US-CNT-2300092
Important Product Information
Caution: Federal (USA) law restricts this device to sale by, or on the order of, a physician.
As part of a properly maintained surgical environment, it is recommended that a backup IOL injector be made available in the event the AutoSert® IOL Injector Handpiece does not perform as expected.
Indication: The CENTURION® Vision system is indicated for emulsification, separation, irrigation, and aspiration of cataracts, residual cortical material and lens epithelial cells, vitreous aspiration and cutting associated with anterior vitrectomy, bipolar coagulation, and intraocular lens injection. The AutoSert® IOL Injector Handpiece is intended to deliver qualified AcrySof® intraocular lenses into the eye following cataract removal.
The AutoSert® IOL Injector Handpiece achieves the functionality of injection of intraocular lenses. The AutoSert® IOL Injector Handpiece is indicated for use with the AcrySof® lenses SN6OWF, SN6AD1, SN6AT3 through SN6AT9, as well as approved AcrySof® lenses that are specifically indicated for use with this inserter, as indicated in the approved labeling of those lenses.
Warnings: Appropriate use of CENTURION® Vision System parameters and accessories is important for successful procedures. Use of low vacuum limits, low flow rates, low bottle heights, high power settings, extended power usage, power usage during occlusion conditions (beeping tones), failure to sufficiently aspirate viscoelastic prior to using power, excessively tight incisions, and combinations of the above actions may result in significant temperature increases at incision site and inside the eye, and lead to severe thermal eye tissue damage.
Good clinical practice dictates the testing for adequate irrigation and aspiration flow prior to entering the eye. Ensure that tubings are not occluded or pinched during any phase of operation.
The consumables used in conjunction with ALCON® instrument products constitute a complete surgical system. Use of consumables and handpieces other than those manufactured by Alcon may affect system performance and create potential hazards.
AEs/Complications: Inadvertent actuation of Prime or Tune while a handpiece is in the eye can create a hazardous condition that may result in patient injury. During any ultrasonic procedure, metal particles may result from inadvertent touching of the ultrasonic tip with a second instrument. Another potential source of metal particles resulting from any ultrasonic handpiece may be the result of ultrasonic energy causing micro abrasion of the ultrasonic tip.
ATTENTION: Refer to the Directions for Use for the accessories/consumables and Operator’s Manual for a complete listing of indications, warnings, cautions and notes.
1. Osher, R. H. Slow motion phacoemulsification approach. J Cataract Refract Surg 19, 667, doi:10.1016/s0886-3350(13)80025-9 (1993).
2. Osher, R. H. Slow-Motion Phacoemulsification. Operative Techniques in Cataract and Refractive Surgery 1, 42-46 (1998).
3. Vasavada, A. R. et al. Impact of high and low aspiration parameters on postoperative outcomes of phacoemulsification: randomized clinical trial. J Cataract Refract Surg 36, 588-593, doi:10.1016/j.jcrs.2009.11.009 (2010).
4. Kokubun, T. et al. in The 126th Annual Meeting of the Japanese Ophthalmological Society (JOS) (Osaka, Japan, 2022).
5. Rauen, M. in American Association of Cataract and Refractive Surgery (ASCRS) Annual Meeting (San Diego, US, 2023).
6. Scarfone, H. A. & Rodriguez, E. C. C. in American Association of Cataract and Refractive Surgery (ASCRS) Annual Meeting (San Diego, US, 2023).
7. Beres, H., de Ortueta, D., Buehner, B. & Scharioth, G. B. Does low infusion pressure microincision cataract surgery (LIPMICS) reduce frequency of post-occlusion breaks? Romanian Journal of Ophthalmology 66, 135-139 (2022).
8. Suzuki, H., Igarashi, T. & Takahashi, H. Effect of a new phacoemulsification and aspiration handpiece on anterior chamber stability. J Cataract Refract Surg 49, 91-96, doi:10.1097/j.jcrs.0000000000001071 (2023).
9. US-CNT-200014. (06/2020).
10. Lim, D. H., Shin, D. H., Han, G., Chung, E. S. & Chung, T. Y. The Incidence and Risk Factors of Lens-iris Diaphragm Retropulsion Syndrome during Phacoemulsification. Korean J Ophthalmol 31, 313-319, doi:10.3341/kjo.2016.0050 (2017).
11. Cionni, R. J., Barros, M. G. & Osher, R. H. Management of lens-iris diaphragm retropulsion syndrome during phacoemulsification. J Cataract Refract Surg 30, 953-956, doi:10.1016/j.jcrs.2004.01.030 (2004).
12. Wilbrandt, H. R. & Wilbrandt, T. H. Pathogenesis and management of the lens-iris diaphragm retropulsion syndrome during phacoemulsification. J Cataract Refract Surg 20, 48-53, doi:10.1016/s0886-3350(13)80043-0 (1994).
13. Anisimova, N. S. et al. Anterior vitreous detachment: risk factor for intraoperative complications during phacoemulsification. J Cataract Refract Surg 46, 55-62, doi:10.1016/j.jcrs.2019.08.005 (2020).
14. Zhang, Z. et al. Incidence and Risk Factors for Berger’s Space Development after Uneventful Cataract Surgery: Evidence from Swept-Source Optical Coherence Tomography. J Clin Med 11, doi:10.3390/jcm11133580 (2022).
15. Lin, W., Luo, J., Li, P., Ji, M. & Guan, H. Anterior vitreous detachment and retrolental material during cataract surgery: incidence and risk factors, with pathological evidence. J Cataract Refract Surg 49, 578-583, doi:10.1097/j.jcrs.0000000000001156 (2023).
16. Vasavada, V. et al. Impact of fluidic parameters during phacoemulsification on the anterior vitreous face behavior: Experimental study. Indian J Ophthalmol 67, 1634-1637, doi:10.4103/ijo.IJO_465_19 (2019).
17. Hilford, D., Hilford, M., Mathew, A. & Polkinghorne, P. J. Posterior vitreous detachment following cataract surgery. Eye (Lond) 23, 1388-1392, doi:10.1038/eye.2008.273 (2009).
18. Liu, J. et al. Microvascular Changes in Macular Area After Phacoemulsification and Its Influencing Factors Assessed by Optical Coherence Tomography Angiography. Ther Clin Risk Manag 17, 405-414, doi:10.2147/TCRM.S309679 (2021).
19. Chen, D. et al. Effect of simulated dynamic intraocular pressure on retinal thickness measured by optical coherence tomography after cataract surgery. Int J Ophthalmol 5, 687-693, doi:10.3980/j.issn.2222-3959.2012.06.07 (2012).
20. Wang J., Tang K. & J., Z. in Asia-Pacific Association for Cataract & Refractive Surgeons (APACRS) (Singapore, 2021).
21. Rauen, M. in American Association of Cataract and Refractive Surgery (ASCRS) Annual Meeting (San Diego, US, 2023).
22. Hou, C. H., Lee, J. S., Chen, K. J. & Lin, K. K. The sources of pain during phacoemulsification using topical anesthesia. Eye (Lond) 26, 749-750, doi:10.1038/eye.2012.29 (2012).
23. Suzuki, H., Oki, K., Shiwa, T., Oharazawa, H. & Takahashi, H. Effect of bottle height on the corneal endothelium during phacoemulsification. J Cataract Refract Surg 35, 2014-2017, doi:10.1016/j.jcrs.2009.05.057 (2009).
24. Vasavada, V. et al. Real-time dynamic intraocular pressure fluctuations during microcoaxial phacoemulsification using different aspiration flow rates and their impact on early postoperative outcomes: a randomized clinical trial. J Refract Surg 30, 534-540, doi:10.3928/1081597X-20140711-06 (2014).
25. Kawasaki, S. et al. Influence of elevated intraocular pressure on the posterior chamber-anterior hyaloid membrane barrier during cataract operations. Arch Ophthalmol 129, 751-757, doi:10.1001/archophthalmol.2011.115 (2011).
26. Zhao, Z., Wen, W., Jiang, C. & Lu, Y. Changes in macular vasculature after uncomplicated phacoemulsification surgery: Optical coherence tomography angiography study. J Cataract Refract Surg 44, 453-458, doi:10.1016/j.jcrs.2018.02.014 (2018).
27. Nourinia, R. et al. Optical coherence tomography angiography parameters after cataract surgery. Int Ophthalmol 43, 2679-2686, doi:10.1007/s10792-023-02667-5 (2023).




