This content is for U.S. healthcare professionals only.
Indications and Usage
DURYSTA™ (bimatoprost implant) is indicated for the reduction of intraocular pressure (IOP) in patients with open angle glaucoma (OAG) or ocular hypertension (OHT).
INTRODUCTION
Glaucoma is a progressive disease estimated to affect approximately 76 million people globally.1 One retrospective study showed that cumulative incidence of blindness in at least 1 eye was 27% after 10 years.2 Elevated intraocular pressure (IOP) is the main risk factor in glaucoma, and the only modifiable factor that has been shown to decrease the risk of visual field loss associated with glaucoma.3,4 A large body of clinical evidence demonstrates that there are a number of efficacious therapies to lower IOP in patients with glaucoma.3 However, the Early Manifest Glaucoma Trial (EMGT) showed 59% of patients (n = 129) progressed.5 This could be due to a lack of IOP control related to a host of factors including administration errors or compliance.
A panel of ophthalmologists recently convened to discuss the changing landscape of IOP reduction in glaucoma, both in terms of treatment options available and how physicians are using them.
THE ROLE OF IOP IN DISEASE PROGRESSION
Nathan Radcliffe, MD: Glaucoma has been a very exciting segment of ophthalmology the last few years. We have seen numerous developments in treatment options, improving our ability to treat each patient with a tailored approach. However, the reported proportion of glaucomatous eyes progressing at faster than -1.5 dB/year varies from 3% to 17% in some studies, while other reports indicate 15% to 20% of eyes manifest visual field index loss greater than 5% per year.6 So, while it’s great that we have effective topical treatments and other interventions, some patients may still progress. What are some of the other possible reasons why patients are still progressing? One of the reasons may be because their IOP is not actually being controlled. What are some of the factors why their IOP may not be under control?
Ehsan Sadri, MD: It is evident that patients fall into different categories of progression. There are some who remain very stable, while others continue to have moderate or aggressive progression. I question how much logistics and medication access have to do with a lack of IOP control.
Preeya K. Gupta, MD: Efficacy with topical IOP-lowering therapy requires strict adherence, and, based on a retrospective analysis of pharmacy claims, adherence rates in topical IOP-lowering therapies are among the lowest in chronic diseases, including cardiovascular and diabetes therapies.7,8 Factors such as complicated treatment regimens, improper drop instillation technique, or not shaking the bottle of liquid suspension can all disrupt proper medication usage and result in worse outcomes.7,9,10 Compounding the problem, patients themselves and their physicians are often unaware of the extent of noncompliance11; one study of 100 patients showed that patients who are less than 80% adherent according to Medication Events Monitoring System (MEMS) devices have worse visual fields.12
Jim Katz, MD: One of the greatest burdens our patients face is medication management. There are several reasons why a patient may not be taking their medications as directed, but as more topical therapies are added to a patient’s regimen, compliance could potentially decrease further. Our current strategy for addressing adherence is to improve our communication with patients and to consider other alternative strategies when appropriate.
Dr. Radcliffe: Thank you for those insights, are there any other considerations for why glaucoma patients may progress?
Inder Paul Singh, MD: One additional consideration is that IOP is a dynamic parameter that has both daily and long-term fluctuations that may not be detected during office hours.13 The Advanced Glaucoma Intervention Study (AGIS) showed that over 100 months, patients have a higher risk of visual field progression if they have higher fluctuations in IOP. Caprioli and colleagues looked at the same data and correlated progression to long-term fluctuation.14 While there is debate regarding the association between progression and IOP fluctuation, these results indicate that long-term IOP fluctuation could be a contributing factor.
Interventional Approaches to Lowering IOP
Dr. Radcliffe: For a number of years, most IOP-lowering treatments beyond topical drops were reserved for more moderate to severe cases. With the bevy of treatments available that have demonstrated efficacy, surgeons are more comfortable adopting them earlier in the treatment process for appropriate patients. With these tools in mind, a number of physicians are now turning to a more proactive approach. We are finding that interventional treatment means that fewer patients are escaping therapy.
Thomas Samuelson, MD: We have always known that when a patient leaves our office, our influence over their therapy is tenuous; compliance is always a concern. With this in mind, I think the recent LIGHT study is a landmark study. The trial demonstrated that over a 3-year period, selective laser trabeculoplasty (SLT) showed no significant difference from the eye drop group. Based on these results, the study indicated that an interventional approach can be offered as a potential first-line treatment.15 This gives us confidence that there appears to be a place for interventional therapies earlier in the treatment process.
Dr. Sadri: While drops are effective and a mainstay of treatment, one study of 62 patients showed that adherence was lower in the 2-drug regimen versus the 1-drug regimen.16 This might be another reason to consider interventional therapies for patients experiencing issues taking their medications as directed.
Overview of DURYSTA (bimatoprost implant)
Dr. Radcliffe: That was interesting commentary on our current treatment options and our overall approach to controlling IOP. DURYSTA, a sustained-release biodegradable implant of 10 mcg bimatoprost, is approved by the US Food and Drug Administration for the reduction of IOP in patients with open-angle glaucoma or ocular hypertension. What are the benefits of this type of approach for IOP control and what are the benefits and risks that we see in the initial data?
Rajesh Rajpal, MD: DURYSTA is a novel, biodegradable, intracameral implant that rests in the inferior angle, providing targeted delivery to the diseased tissues and consistent IOP control for several months (Figure 1).17
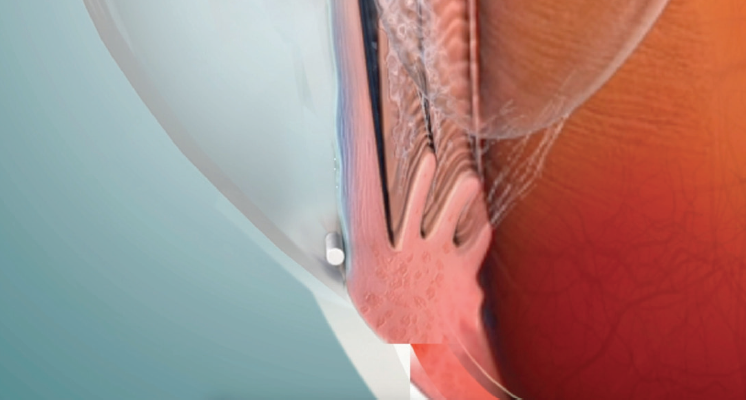
Figure 1. DURYSTA is a novel, biodegradable, intracameral implant that rests in the inferior angle.
Dr. Sadri: DURYSTA is the first pharmaceutical treatment option for IOP lowering in glaucoma where the physician is able to administer what the patient actually receives. Before, when a patient would go to the pharmacy, there was a chance they would receive a generic substitution. Even when patients receive the intended medication, there is no way of ensuring that the patient is instilling it correctly. Now, we know what they are getting and when. DURYSTA is administered intracamerally using a single-use 28-gauge application needle to deliver the biodegradable implant, which will dissolve over time.17
Getting to know DURYSTA™
Iqbal Ike K. Ahmed, MD, FRCSC, and Nathan Radcliffe, MD, discuss DURYSTA™ and what they feel are the most compelling features of the product.
Dr. Radcliffe: Let’s talk about the phase 3 clinical trials. The ARTEMIS 1 and ARTEMIS 2 multicenter, randomized, parallel-group, controlled studies compared 10 mcg bimatoprost implant to twice-daily timolol 0.5% topical drops (Figure 2). Parallel groups of patients diagnosed with open-angle glaucoma or ocular hypertension with a baseline IOP of 22 to 32 mm Hg at hour 0 after washout were followed for a period of 20 months, including an 8-month extended follow-up.17
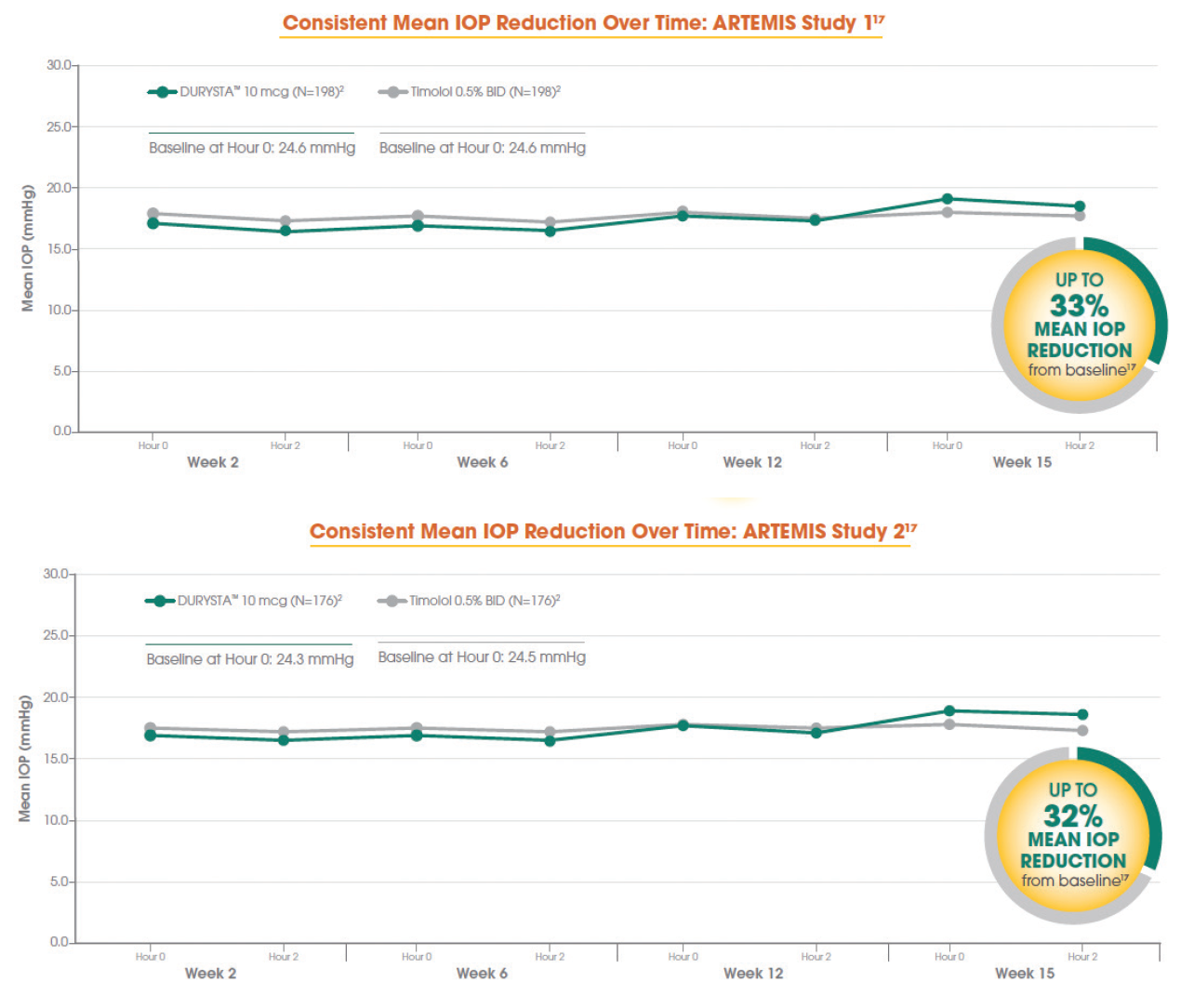
Figure 2. Data from the ARTEMIS 1 Trial (top) and the ARTEMIS 2 Trial (bottom).
Dr. Gupta: As you can see in Figure 2, the phase 3 data show that DURYSTA (bimatoprost implant) lowered IOP by up to 33% from baseline over the 12-week primary efficacy period. This works out to be about 5 to 8 mm Hg of reduction from the mean baseline IOP of 24.5 mm Hg.17
Dr. Samuelson: When interpreting the phase 3 data from the ARTEMIS trials, there is compelling reason to choose it among the initial treatment options. In the ARTEMIS studies, DURYSTA provided sustained IOP control for several months.17
Dr. Gupta: Anytime there is a device or medication released, we look for efficacy but also safety. What was the incidence of the most common side effects in the ARTEMIS trials?
Dr. Sadri: The most common ocular adverse reaction was conjunctival hyperemia, which occurred in 27% of patients. Other common ocular adverse reactions reported in 5% to 10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, IOP increased, corneal endothelial cell loss, vision blurred, and iritis.17
Ocular adverse reactions occurring in 1% to 5% of patients were anterior chamber cell, lacrimation increased, corneal edema, aqueous humor leakage, iris adhesions, ocular discomfort, corneal touch, iris hyperpigmentation, anterior chamber flare, anterior chamber inflammation, and macular edema.17
The most common nonocular adverse event was headache, which was observed in 5% of patients.17
Anytime something is implanted inside the eye, our primary concern is usually infection. Proper aseptic technique must always be used with administering DURYSTA, and patients should be monitored following administration. While the trials demonstrated no incidence of endophthalmitis, these types of intraocular procedures and injections have been associated with endophthalmitis and patients should be monitored following the administration.17
Dr. Gupta: As a cornea specialist, I’m really excited about a pressure-lowering medication with administration that bypasses the ocular surface. The results of the trials give me confidence that I can deliver an implant directly to targeted tissues.
DURYSTA™ Safety and Patient Selection
Iqbal Ike K. Ahmed, MD, FRCSC, and Nathan Radcliffe, MD, share their thoughts on the safety profile of DURYSTA™, the importance of patient selection, and where DURYSTA™ will fit in their current treatment paradigms.
PATIENT TYPES
Dr. Radcliffe: What are we trying to do for our patients when we initiate therapy? I would say that a patient who needs IOP lowering and maybe could benefit from a preservative-free treatment would be a great candidate for this sustained-release implant (Figure 3). It’s important to note that DURYSTA is for single administration only and should not be re-administered to an eye that received a prior DURYSTA. Which other patients are ideal candidates for DURYSTA?

Figure 3. This image depicts the biodegradable sustained-release DURYSTA implant on a dime.
Dr. Singh: There is a wide scope for application of this therapy. My first thought is any patient for whom drops are not suitable: inability to instill drops, forgetfulness, cost, etc. Anyone who has difficulty applying drops, for whatever reason, could be a candidate for DURYSTA. I also feel this could help provide another option to achieve the necessary lowering of IOP while considering our next course of action for a patient.
The Versatility of DURYSTA™
Inder Paul Singh, MD, and Preeya K. Gupta, MD, discuss treatment options and where they'll use DURYSTA™ in their practice.
Dr. Radcliffe: It takes 2 people to be noncompliant. The patient has to be noncompliant with their drops, and then the doctor has to be complicit by not addressing compliance with the patient or not recommending another option. It used to be that my only option other than topical drops was SLT. Now when I have a patient who needs IOP lowering and either does not want or is not a good candidate for topical drops, I can offer them SLT or DURYSTA (bimatoprost implant). This is also a good option for patients who have previously had SLT.
DURYSTA™ and other Interventions
Iqbal Ike K. Ahmed, MD, FRCSC, and Nathan Radcliffe, MD, discuss DURYSTA™ and other treatment modalities.
Dr. Katz: When a patient is sensitive to the preservative of a topical medication, DURYSTA could be a suitable option. For patients on multiple medications, if I need to stop those treatments for whatever reason, I can administer DURYSTA, before deciding what to do next.
Dr. Sadri: A patient on multiple drops who is now looking for an alternative treatment option to lower their IOP would be a potential candidate for DURYSTA. You get the benefit of knowing that the therapeutic drug is being directly delivered to the diseased tissue for consistent IOP control for several months. As a reminder, DURYSTA should not be re-administered to an eye that received a prior DURYSTA.
Dr. Radcliffe: The label indicates that administering DURYSTA must be done under magnification that allows clear visualization of the anterior chamber and the patient’s head must be stabilized.17 The complete administration instructions can be found in the accompanying full Prescribing Information. That said, what are some other considerations around administering DURYSTA?
Dr. Singh: Surgeons can choose to perform the implantation in an office exam room setting, a minor procedure room, a hospital, or an ASC.
Dr. Samuelson: When performing this procedure, it is necessary to be aware of the vital structures present: the corneal endothelium, the iris, and the lens. You want to make sure that the implant lies nicely in the inferior angle and be mindful to retract the needle slowly. You also want to make sure that the wound is self-sealing, so good technique should be stressed.
Dr. Gupta: Getting comfortable with the implant and with the visualization is key. Wherever the physician decides to perform the implantation, standard aseptic technique should always be used.
DURYSTA AND THE PATIENT PERSPECTIVE
Dr. Gupta: How would you describe DURYSTA to patients?
Dr. Singh: We need to help patients understand the risks and benefits of DURYSTA, particularly that it is preservative free, and it is physician administered so they don’t have to remember to take it every day. It will be necessary to confidently present the safety profile and the benefits of targeted delivery to the diseased tissues within the anterior chamber and sustained IOP control for several months. When properly educated, patients can make informed decisions about their disease management.
Dr. Rajpal: I will focus on sustained IOP control as this is something patients easily identify with. It is similar to diabetes management, where the goal is to keep blood sugar steady. Patients will intuitively be able to understand that when they have something in their eye dispensing a little bit of a drug 24/7 for several months, it will help control their IOP. Their understanding of disease management may improve.
Dr. Singh: There is a certain element of fear we can induce by telling a patient, “I’m going to inject this implant in your eye.” It is very important to appropriately describe the procedure to patients. When I perform a laser treatment such as SLT, I don’t say, “I’m going to shoot a laser beam into your eye that is going to drop your pressure.” I say, “There’s a beam of light that opens up your natural drainage pathway.” In a similar fashion, with DURYSTA I would say, “I’m going to place this tiny dissolvable implant inside your eye that will release medicine over time, so you don’t have to remember to take your medication every day.” The confidence and belief in the products are what patients respond to.
Talking to Patients about DURYSTA™
Inder Paul Singh, MD, and Preeya K. Gutpa, MD, describe how they present and recommend DURYSTA™ to patients.
Dr. Sadri: My greatest goal when utilizing an interventional approach is to find ways that control patients’ IOP, without the patient having to administer the medication on their own. With intervention, we can provide targeted therapy that lends itself to what we are trying to do as ophthalmologists.
Dr. Samuelson: I believe that a barrier to care is the physician mindset, and it’s time we started changing that. There are a lot of reasons to engage in interventional glaucoma care, and there are distinct risks and benefits to an interventional approach. We have a luxury of choices these days, but we need to own them. If we simply list off all the options to our patients, they will not know which way to turn. We need to tell them that while the traditional standard of care is effective, there is high-quality evidence that there are alternatives that should also be considered. The first physician-administered, intracameral implant to lower IOP represents a paradigm shift in how we deliver medication to glaucoma patients.
Dr. Singh: DURYSTA (bimatoprost implant) demonstrates sustained IOP lowering with an established safety profile. Modern patient care must consider a multitude of factors. I often ask, are we doing everything we can to control a patient’s IOP? Having the ability to decrease IOP with an intracameral implant is a true game changer.
DURYSTA™ Indications and Usage and Important Safety Information
Indications and Usage
DURYSTA™ (bimatoprost implant) is indicated for the reduction of intraocular pressure (IOP) in patients with open angle glaucoma (OAG) or ocular hypertension (OHT).
Important Safety Information
Contraindications
DURYSTA™ (bimatoprost implant) is contraindicated in patients with: active or suspected ocular or periocular infections; corneal endothelial cell dystrophy (e.g., Fuchs’ Dystrophy); prior corneal transplantation or endothelial cell transplants (e.g., Descemet’s Stripping Automated Endothelial Keratoplasty [DSAEK]); absent or ruptured posterior lens capsule, due to the risk of implant migration into the posterior segment; hypersensitivity to bimatoprost or to any other components of the product.
Warnings and Precautions
The presence of DURYSTA™ implants has been associated with corneal adverse reactions and increased risk of corneal endothelial cell loss. Administration of DURYSTA™ should be limited to a single implant per eye without retreatment. Caution should be used when prescribing DURYSTA™ in patients with limited corneal endothelial cell reserve.
DURYSTA™ should be used with caution in patients with narrow iridocorneal angles (Shaffer grade 3) or anatomical obstruction (e.g., scarring) that may prohibit settling in the inferior angle.
Macular edema, including cystoid macular edema, has been reported during treatment with ophthalmic bimatoprost, including DURYSTA™ intracameral implant. DURYSTA™ should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Prostaglandin analogs, including DURYSTA™, have been reported to cause intraocular inflammation. DURYSTA™ should be used with caution in patients with active intraocular inflammation (e.g., uveitis) because the inflammation may be exacerbated.
Ophthalmic bimatoprost, including DURYSTA™ intracameral implant, has been reported to cause changes to pigmented tissues, such as increased pigmentation of the iris. Pigmentation of the iris is likely to be permanent. Patients who receive treatment should be informed of the possibility of increased pigmentation. While treatment with DURYSTA™ can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly.
Intraocular surgical procedures and injections have been associated with endophthalmitis. Proper aseptic technique must always be used with administering DURYSTA™, and patients should be monitored following the administration.
Adverse Reactions
In controlled studies, the most common ocular adverse reaction reported by 27% of patients was conjunctival hyperemia. Other common adverse reactions reported in 5%-10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, intraocular pressure increased, corneal endothelial cell loss, vision blurred, iritis, and headache.
Please click here for full Prescribing Information.
© 2020 AbbVie. All rights reserved. DURYSTA™ and its design are trademarks of Allergan, Inc., an AbbVie company. DurystaHCP.com DUR141573 12/20
1. Tham Y, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-2090.
2. Peters D, Bengtsson B, Heijl A. Lifetime risk of blindness in open-angle glaucoma. Am J Ophthalmol. 2013;156(4):724-730.
3. American Academy of Ophthalmology. Preferred Practice Pattern® Guidelines. Primary open-angle glaucoma. San Francisco, CA: American Academy of Ophthalmology. 2015.
4. Coleman AL, Kodjebacheva G. Risk factors for glaucoma needing more attention. Open Ophthalmol J. 2009;3:38-42.
5. Leske MC, Heijl A, Hyman L, et al; EMGT Group. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114(11):1965-1972.
6. Saunders LJ, Medeiros FA, Weinreb RN, et al. What rates of glaucoma progression are clinically significant? Expert Rev Ophthalmol. 2016;11(3):227-234.
7. Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(suppl 1):S57-S68.
8. Yeaw J, Benner JS, Walt JG, et al. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728-740.
9. Gomes BF, Paredes AF, Madeira N, et al. Assessment of eye drop instillation technique in glaucoma patients. Arq Bras Oftalmol. 2017;80(4):238-241.
10. Gao X, Yang Q, Huang W, et al. Evaluating eye drop instillation technique and its determinants in glaucoma patients. J Ophthalmol. 2018;2018:1376020.
11. Stone JL, Robin AL, Novack GD, et al. An objective evaluation of eyedrop instillation in patients with glaucoma. Arch Ophthalmol. 2009;127(6):732-736.
12. Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011;118(12):2398-2402.
13. Barkana Y, Anis S, Liebmann J, et al. Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol. 2006;124(6):793-797.
14. Caprioli J, Coleman AL. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the Advanced Glaucoma Intervention Study. Ophthalmology. 2008;115(7):1123-1129.e3.
15. Gazzard G, Konstantakopoulou E, Garway-Heath D, et al; LiGHT Trial Study Group. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): A multicentre randomised controlled trial. Lancet. 2019;393(10180):1505-1516.
16. Robin AL, Novack GD, Covert DW, et al. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144(4):533-540.
17. DURYSTA Prescribing Information.
DURYSTA™ Important Safety Information for Healthcare Professionals
Collapse -
Important Safety Information
Contraindications
DURYSTA™ (bimatoprost implant) is contraindicated in patients with: active or suspected ocular or periocular infections; corneal endothelial cell dystrophy (e.g., Fuchs' Dystrophy); prior corneal transplantation or endothelial cell transplants (e.g., Descemet's Stripping Automated Endothelial Keratoplasty [DSAEK]); absent or ruptured posterior lens capsule, due to the risk of implant migration into the posterior segment; hypersensitivity to bimatoprost or to any other components of the product.
Warnings and Precautions
The presence of DURYSTA™ implants has been associated with corneal adverse reactions and increased risk of corneal endothelial cell loss. Administration of DURYSTA™ should be limited to a single implant per eye without retreatment. Caution should be used when prescribing DURYSTA™ in patients with limited corneal endothelial cell reserve.
DURYSTA™ should be used with caution in patients with narrow iridocorneal angles (Shaffer grade 3) or anatomical obstruction (e.g., scarring) that may prohibit settling in the inferior angle.
Macular edema, including cystoid macular edema, has been reported during treatment with ophthalmic bimatoprost, including DURYSTA™ intracameral implant. DURYSTA™ should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Prostaglandin analogs, including DURYSTA™, have been reported to cause intraocular inflammation. DURYSTA™ should be used with caution in patients with active intraocular inflammation (e.g., uveitis) because the inflammation may be exacerbated.
Ophthalmic bimatoprost, including DURYSTA™ intracameral implant, has been reported to cause changes to pigmented tissues, such as increased pigmentation of the iris. Pigmentation of the iris is likely to be permanent. Patients who receive treatment should be informed of the possibility of increased pigmentation. While treatment with DURYSTA™ can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly.
Intraocular surgical procedures and injections have been associated with endophthalmitis. Proper aseptic technique must always be used with administering DURYSTA™, and patients should be monitored following the administration.
Adverse Reactions
In controlled studies, the most common ocular adverse reaction reported by 27% of patients was conjunctival hyperemia. Other common adverse reactions reported in 5%-10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, intraocular pressure increased, corneal endothelial cell loss, vision blurred, iritis, and headache.
Please click here for full Prescribing Information.










