
The EVO ICL lens (EVO; STAAR Surgical; Figure 1) was approved by the FDA in March 2022. In the past 6 months, I have performed more than 200 EVO ICL procedures. My experience with the ICL family of lenses, however, extends well beyond the recent approval. I have been using the Visian ICL technology with excellent results since 2008 and have performed more than 1,000 procedures with both technologies. Just as the ICL has evolved over time, so has the way that I recommend the technology to patients. The new EVO has given me even more confidence to recommend the technology to a wider range of patients.

Figure 1. The EVO ICL.
THEN VERSUS NOW
Then. Before the EVO was approved, I recommended the ICL procedure only to patients with troublesome corneas or high myopia greater than -10.00 D spherical equivalent. My reason was twofold. First, the procedure required a peripheral iridotomy to facilitate the flow of fluid within the eye. This was inconvenient for patients because they had to come in for a separate procedure, there was some discomfort, and they occasionally experienced glare from the peripheral iridotomy. Second, in the FDA clinical trial, there was a 1.3% rate of clinically significant anterior subcapsular cataract reported up to 5 years after the ICL procedure.1

Now. Now, EVO has given me increased confidence in the technology. For starters, there is no longer a need for the preoperative peripheral iridotomy because the EVO is designed with a central port, which lowers the risk of pupillary block and cataract. A literature review of the EVO found no incidence of anterior subcapsular cataracts between 1 and 5 years postoperatively that were attributed to the implantation of the lens.2 And, in my own personal experience, fewer than 1% of eyes have IOP greater than 25 mm Hg in the first 24 hours.
For these reasons, I recommend the EVO to more patients than ever before, including those with a lower amount of myopia than I would have treated previously. Currently, my practice strongly recommends the EVO for patients up to the age of 45 years old with myopia greater than -8.00 D spherical equivalent; patients with less than 280 µm of calculated residual stromal bed after LASIK, SMILE, or PRK; and patients with higher risk of keratoconus based on topography or epithelial mapping. We also offer EVO for patients who meet the definition of high myopia (-6.00 D) if they want better quality vision, and we’ve implanted the EVO for as low as -3.00 D of myopia.
The more procedures I do, the more confident I am recommending the technology. And it’s not just me—all of our counselors and our whole team are so excited to see patients qualify for EVO surgery because we know that they will be happy the next day. Most patients are 20/20 or better uncorrected the next day (Figure 2).
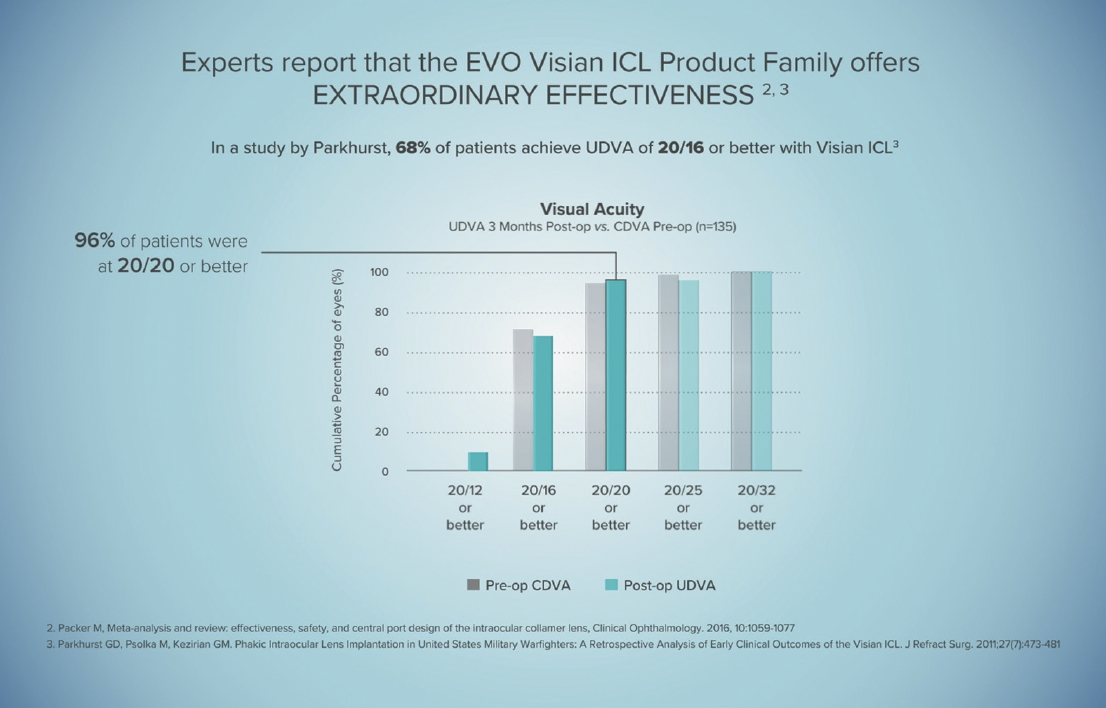
Figure 2. Most patients who receive the EVO Visian ICL achieve 20/20 or better UDVA.
Having confidence in the procedure you are recommending is key. Patients pick up on that, and they feel reassured that they are being recommended the best procedure based on their unique case. As we treat more patients, and the technology is more known to the public, more patients will come in asking for EVO in the United States, like in other countries.
THE PATIENT CONVERSATION
Generally, patients are comfortable with the EVO procedure once we recommend the treatment to them and share its advantages. They feel reassured that I can remove the EVO, if necessary, and that our removal rate is extremely low. We also explain to patients that the Collamer material has been used in the eye for more than 30 years with excellent biocompatibility of the material.* For patients with myopia greater than -6.00 D, we tell them that based on our patients’ experiences, they will likely have better day and nighttime vision compared to LASIK, SMILE, and their current glasses or soft contact lenses. Lastly, we tell patients that, because there is no removal of corneal tissue with the EVO, they will be candidates for more IOL options in the future when they need cataract surgery.

In the case of refractive surgery, it is important for the surgeon to make a strong recommendation for the best procedure for that patient. In my experience, this is more impactful to patients and preferable to telling them that they can do either. The latter confuses them, and they may choose the lower-priced option or the one they are more familiar with.
When patients understand that the EVO is not only a safe and effective procedure but one that also gives them the most options for the future, they feel confident in their decision to proceed with EVO surgery. When strong candidates for the EVO are deciding between that procedure and LASIK, for instance, I like to say something akin to the following: “The EVO is the recommended procedure for you. Both are good, but this is strongly recommended because the EVO preserves the cornea and will leave you with more IOL options in the future. I can remove it, if needed, and will likely give you excellent quality of vision.”
I also find it helpful to involve patients in the decision to learn more. For example, if someone comes in for LASIK but I feel they are a better candidate for the EVO, I acknowledge their viewpoint and say, “I know you came in for LASIK. It’s a great procedure, but would you like to hear about something that may be even better suited for your eyes?” Now, they’ve given you permission to tell them about an alternative technology, and they are more likely to trust you and your staff when you tell them they are a better candidate for EVO. This is called the yes-yes technique, and it has been effective in my practice. The patient must feel that you only want what’s best for them. Be confident with your words, use appropriate body language, and speak with a gentle tone of voice.
Patients who show up at your office are typically motivated to achieve spectacle independence, but they still need help deciding what procedure is right for them. By following the pointers outlined above, you can learn to have a successful dialogue with patients and position the EVO as the recommended treatment for a growing range of patients.
1. Sanders DR. Anterior subcapsular opacities and cataracts 5 years after surgery in the Visian implantable collamer lens FDA trial. J Refract Surg. 2008; 24:566–70.
2. Packer M. The Implantable Collamer Lens with a central port: review of the literature. Clin Ophthalmol. 2018;12:2427-2438.
