
Rayner, a renowned global leader in innovative ophthalmic solutions and the pioneering manufacturer of the world’s first intraocular lens (IOL) in 1949, proudly announces a significant achievement in their ongoing dedication to improving vision. Last month, the company celebrates the successful implantation of its 4 millionth RayOne IOL, underscoring its commitment to advancing eye care worldwide.
- Rayner celebrates milestone with successful implantation of 4 millionth RayOne IOL, following the recent achievement of 10 million IOL implantations made with Rayacryl.
- Rayner continues to innovate and expand global footprint with the growth of RayOne EMV.
Introduced in 2016, RayOne is Rayner’s fully preloaded IOL system, boasting an intuitive two-step injector and a 1.65-mm nozzle diameter. With nine available optics*, including monofocal, toric, enhanced monofocal, and trifocal options, RayOne captured international acclaim in 2020 when it was honored with The Queen’s Awards for Enterprise (Innovation).
This RayOne IOL milestone follows the recent achievement of 10 million IOL implantations made with Rayacryl, Rayner’s hydrophilic material renowned for its glistening-free properties, which serves as the foundation for both legacy and most RayOne IOLs.
The distinction of performing the 4 millionth RayOne IOL implantation fell to Dr. Nathan Radcliffe of New York Eye in New York, New York, USA.** Dr. Radcliffe, a long-standing advocate of Rayner lenses, expressed his satisfaction with RayOne IOLs and its benefits for his patients and practice (Figure). “As a surgeon, it has been a pleasure to bring the RayOne technology to my patients and to my surgery center. We have all been extremely pleased with the experience and outcomes and knowing that my peers and I have now implanted 4 million RayOne lenses confirms to me that I am not alone in recognizing the value of this fantastic IOL technology.”
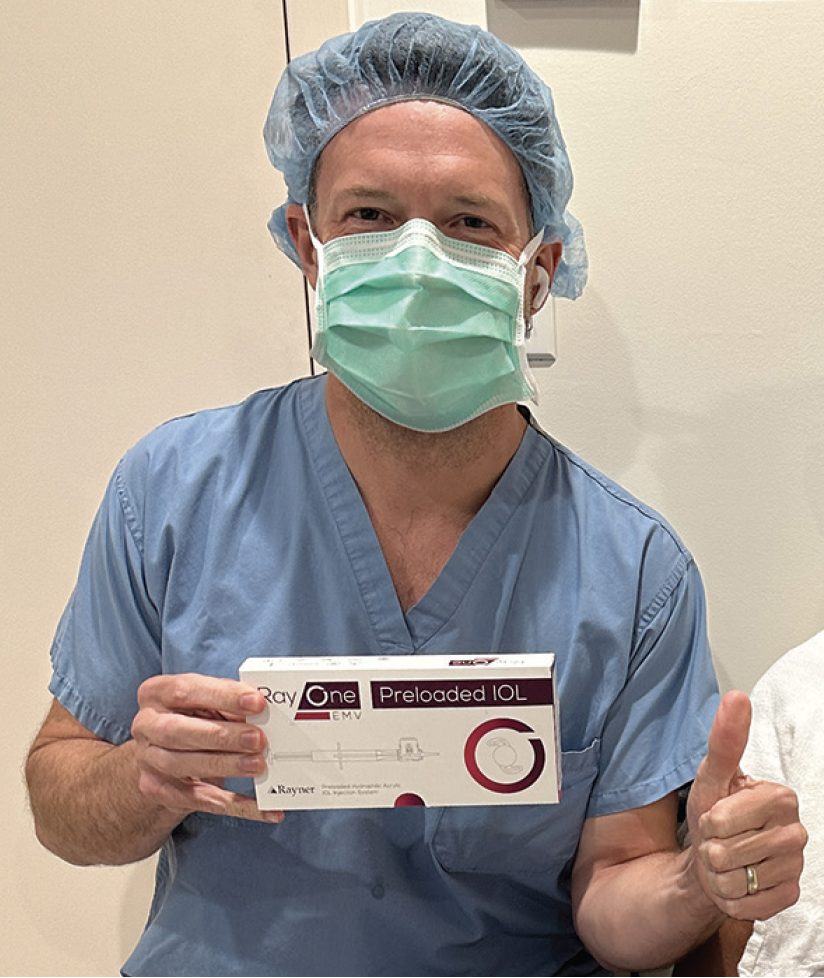
Figure. Dr. Radcliffe with RayOne EMV, the 4 millionth RayOne lens implanted.
As the global demand for cutting-edge eye care solutions continues to grow, Rayner remains unwavering in its dedication to spearheading breakthrough technologies. It’s no coincidence that RayOne EMV, Rayner’s latest IOL innovation and an enhanced monofocal developed in partnership with Prof. Graham Barrett, was the 4 millionth lens to be implanted.
“Given the rapid growth of the RayOne platform and RayOne EMV in particular, I can see that we are just at the beginning of the success of this fantastic surgical option for cataract patients,” elaborated Dr. Radcliffe.
“As a surgeon, it has been a pleasure to bring the RayOne technology to my patients and to my surgery center. We have all been extremely pleased with the experience and outcomes and knowing that my peers and I have now implanted 4 million RayOne lenses confirms to me that I am not alone in recognizing the value of this fantastic IOL technology.” —NATHAN RADCLIFFE, MD
With a robust pipeline of research and development projects, Rayner is well-positioned to introduce additional groundbreaking IOLs in the coming years. The company looks forward to expanding its presence in the global market while upholding its mission of advancing eye care.
About Rayner
Since the implantation of the first Rayner intraocular lens (IOL) by Sir Harold Ridley in 1949, Rayner has continuously pioneered IOL design with the goal to improve vision and restore sight worldwide. Today, Rayner’s mission remains to deliver innovative and clinically superior ophthalmic products that respond to the expectations of our global customers to improve the sight and quality of life of their patients.
Headquartered in Worthing, United Kingdom, Rayner markets its medical devices, pharmaceuticals, and digital solutions worldwide in over 80 countries through a network of distributors and includes direct sales teams in the United Kingdom, USA, Canada, India, Poland, Australia, Germany, Austria, Switzerland, France, Italy, Spain, and Portugal.
Not all Rayner products are approved for sale in every country.
Please contact your local Rayner distributor for details of which products are available in your area.
©2023 Rayner Group, all rights reserved. Rayner and RayOne are proprietary marks of Rayner. All other trademarks are property of their respective owners. Rayner, 10 Dominion Way, Worthing, West Sussex, BN14 8AQ. Registered in England: 615539. EC 2023-88 06/23
For further information and media enquiries, please contact Rayner: marketingteam@rayner.com
*Not all RayOne models are approved for sale in every country.
**Dr. Radcliffe is a paid consultant for Rayner.
