Marguerite B. McDonald, MD, FACS, moderated a panel discussion with Preeya K. Gupta, MD, and Douglas K. Devries, OD, at the 2021 ASCRS Annual Meeting in Las Vegas on treating meibomian gland dysfunction (MGD). The clinicians discussed the needs of their MGD patients and how the Systane iLux Thermal Pulsation System (Alcon) is using technology to bring symptomatic relief, as well as improve meibomian gland function in their patients, including cataract and refractive patients.
The State of Dry Eye Management
Dry eye disease (DED) is believed to affect nearly 30 million people in the United States alone, but only about half that number are diagnosed.1,2 Dr. McDonald questioned the panel regarding the low diagnosis rate, and all agreed that the biggest issue is that clinicians just aren’t looking for dry eye or MGD. Whether it’s because there can be a mismatch between signs and symptoms, or unfamiliarity with the newer diagnostic tools, the biggest obstacle remains integrating dry eye diagnosis into primary or surgical patient care routines. “It takes time to develop pattern recognition and institute the same steps for every patient. So many patients have DED, and until we’re patterned to check all of our patients, we are going to miss it,” said Dr. Gupta.
MGD remains a very common form of OSD (ocular surface disease), with as many as 86% of DED patients having concomitant MGD.3 MGD is the primary cause of evaporative DED. Dr. Devries expressed that, just like DED, MGD remains under-diagnosed. He stated, “In a study of preoperative cataract patients, only 23% had symptoms, yet 75% of patients had clinical signs.4 If we’re not looking at every patient, we are going to miss a lot of MGD and evaporative DED.” Dr. Devries continued, “I really encourage colleagues to press on those meibomian glands and see if they have good meibum or not. It’s that simple.”
MGD Management Benefits Patients
Patients with MGD can be classified into two groups: symptomatic and asymptomatic. The symptomatic patients are looking for relief that is quick and efficacious. “In our modern society, people don’t have time for the symptoms related to MGD,” shared Dr. Gupta. “Hot compresses twice a day and multiple drops take a lot of time, and it’s not realistic to expect our patients who are busy or have an active lifestyle to comply with that.” Dr. McDonald reiterated the need for something to manage MGD that is fast-acting and long-lasting. “Patients who tried older regimens that took too long to create a noticeable difference have given up and stopped treatment.5 They are just suffering through,” she said.
Clinically, doctors that look for MGD do so to treat the condition, in hopes of preventing or slowing progression. “If you don’t treat MGD, it doesn’t get better on its own,” said Dr. Devries. “It becomes more obstructive until the meibum is solidified and totally inspissated. Patients who may have had little to no symptoms initially, may now notice symptoms increase to a point that is disruptive to their lifestyle.”6
Lack of symptoms doesn’t mean that DED should be ignored, particularly in preoperative patients. Epitropoulos and colleagues found significantly more variability in average keratometry and anterior corneal astigmatism in hyperosmolar patients (>316mOsm/L)7, and Matossian reported that treating MGD preoperatively with thermal pulsation therapy changed the planned intervention for astigmatism correction in 40% of patients.8 Asymptomatic patient motivation to follow through with MGD treatment has been historically reduced by the lack of an efficacious and simple treatment. Dr. McDonald recommends showing patients the meibography images and tear osmolarity scores to help emphasize the importance of treatment even in asymptomatic patients. She stated, “Even though they may be asymptomatic now, they likely won’t be in the future. Untreated DED is a spiral downhill and patients will eventually have very significant symptoms that alter their quality of life.”9
Dr. Gupta finds that it is rare for a patient to actually be completely asymptomatic. Rather, it’s more common that they don’t recognize that the symptoms they are experiencing are a result of MGD. “Whether it’s our routine primary care patients or our presurgical patients, the quality of the tear film dictates how well they are going to see,” said Dr. Gupta. “When a patient reports they don’t have any symptoms, I tell them that I also care about their anatomy, and their meibomian glands are essential to maintaining their visual quality.”10
First-line and Mainstay Therapies for MGD
Patients with MGD tend to have increased bacteria on the lid margin11 and changes in meibum composition12,13, which may result in reduced meibum fluidity.14 All of this leads to the need for therapies that are specific to the disease state. “Relieving the obstruction of glands is a fundamental mechanism for treating MGD,” stated Dr. Gupta. “We know that it’s unhealthy meibum resulting in the obstruction. Expression or evacuation of the glands is the first step to actually getting patients to a point where they can develop regular, healthy meibum.”
Dr. Devries agreed with this approach. “When I see a patient who has obstruction of the glands, increased inflammation, and elevated tear osmolarity, I tell them we are going to get them on a comprehensive program, but that it must start by treating their eyelids,” said Dr. Devries. “I love showing them gland atrophy with meibography images and educating them on the impact. It makes such a big impression on our patients’ understanding of the disease process.”
The panel all agreed that cleaning out the glands should be initiated in the office with an effective therapy, rather than relegated to at-home treatments like warm compresses suffering from fickle patient compliance.15 Dr. McDonald is sympathetic to patients who are less than consistent with at-home warm compress treatments. “Who wants to go heat up an eye mask and put it on at the end of the day?” she asked. “Even when there is good compliance, there is variable efficacy with at-home treatments. The in-office heating and expression options are more convenient and more effective.”16 At-home therapies have been reported to vary in how much heat is transmitted, penetrates, and is retained deep inside the eyelid, where the meibomian glands reside.17 Furthermore, without a consistent method for expressing meibomian glands at home, patients risk the meibum regressing to a stiff consistency upon heat removal. As a result, suboptimal and ineffective lid hygiene is commonly practiced and abandoned prematurely as ineffective.5
However, patients who have the meibomian gland obstruction cleared in the office may benefit from using warm compresses and lid scrubs at home as maintenance therapy. Dr. Gupta stresses the importance of a partnership between patients and clinicians. “I tell my patients that I cannot cure their disease process. This is a chronic disease,” she stated. “But once they understand the importance of treatment and maintenance, there is a mindset change.”
The Systane iLux Thermal Pulsation System
MGD patients have stiffer meibum and cooler eyelid temperatures compared to non-MGD patients, requiring supplemental heat to loosen the meibum and clear the blockage.14,18 The optimum temperature to achieve 90% meibum fluidity is 41.5°C.19 Once a therapeutic level of heat transforms the inspissated meibum into a fluid, lid expression evacuates glands and forces the meibum to the lid margin. Over the last decade there has been an expansion in the number of devices to treat MGD in office, and there are currently two commercial devices that simultaneously heat and express the meibomian glands. Dr. McDonald stated that the Systane iLux Thermal Pulsation System (Alcon) is the only system to offer the capability for treatment customization. She explained, “A section of the eyelid is positioned between the inner and outer eyelid pads and then a light-emitting diode transmits light at 568-nm and 850-nm wavelengths.17 The lime-green 568-nm light penetrates the epidermis and dermis layers and is absorbed by oxyhemoglobin, deoxyhemoglobin, and melanin. The near-infrared 850-nm light penetrates deeper into the tissue and is absorbed by the same chromophores. This creates localized heat that brings the glands to a temperature of 40°C-42°C and maintains it there. It is a very tight temperature range that is safe but very effective at liquefying the meibum.19 After 40 seconds of heat, the gland expression begins with the operator applying a customized amount of pressure until the gland is expressed.”
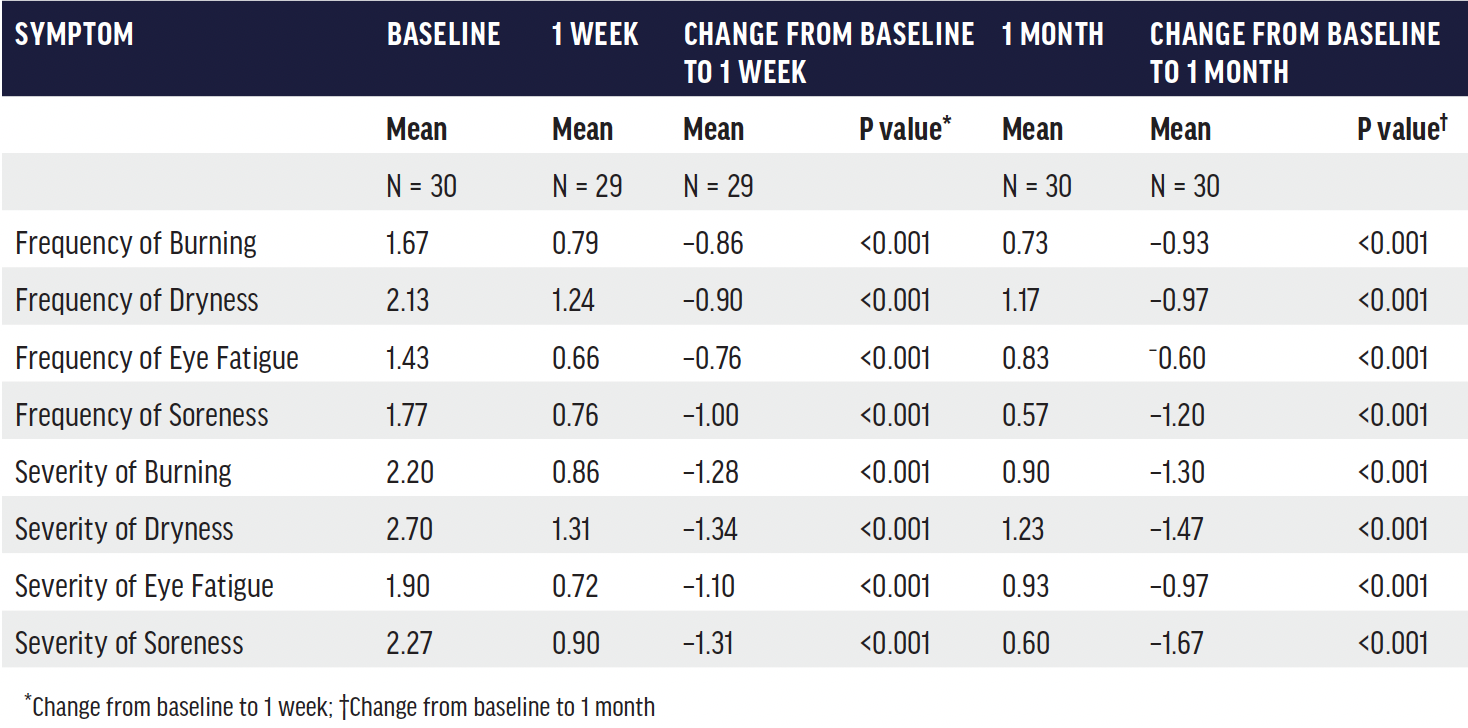
TABLE. Mean and change in sub-scores of Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire at 1 and 4 weeks after a single Systane iLux treatment.20
Treatment with the iLux is much more than simply applying heat and pressure. “When you get into the science involved, you realize how much science went into determining the exact wavelengths of light that the chromophores would absorb,” stated Dr. Devries. “The procedure is very specifically targeting the tissue that needs to be treated.” Dr. Gupta agreed, “This is such an excellent point. Traditional at-home therapies do not target specific tissues. The meibomian glands are in the posterior aspect of the lid, and the variable penetration with different wavelengths of light makes the Systane iLux device different from other treatment options that purely rely on conductive heat processes.”
In addition to the therapeutic heat and the customized pressure, the panel also appreciates the portable footprint. “This is a portable, handheld system, which makes it very unique,” added Dr. McDonald. “You can put it in your briefcase and take it to your other offices very easily. That is an added benefit that makes this a great addition to our armamentarium.”
Systane iLux Clinical Data
Clinical trials of the Systane iLux Thermal Pulsation System reviewed three important outcome measures. The first was meibomian gland secretion (MGS). This was evaluated by applying pressure to the eyelid margin and assessing the quality of the meibum secreted for each of 15 glands on a scale of 0 to 3 (0 = none, 1 = inspissated, 2 = cloudy, and 3 = clear). The total MGS score is the sum of the scores of all 15 glands evaluated, with a maximum score of 45. The second outcome evaluated was tear film stability, which was measured with fluorescein stain. The final outcome measured was symptoms, which was assessed with validated questionnaires such as Ocular Surface Disease Index (OSDI), Standard Patient Evaluation of Eye Dryness (SPEED), and Impact of Dry Eye on Everyday Life – Symptom Bother (IDEEL-SB).20
Dr. McDonald shared the outcomes of a recent study by David Schanzlin, MD.20 “Patients in this study saw significant improvements in all areas,” she said. “Average MGS improved from 4.1 at baseline to 15.8 at 1 week and 18.3 at 1 month. Tear break-up time (TBUT) increased from an average of 5 seconds at baseline to approximately 8 seconds at 1 week. The severity of symptoms improved such that the average patient was re-categorized from severe to mild or borderline mild-moderate (Figure 1 and Table). These are remarkably fast improvements.”
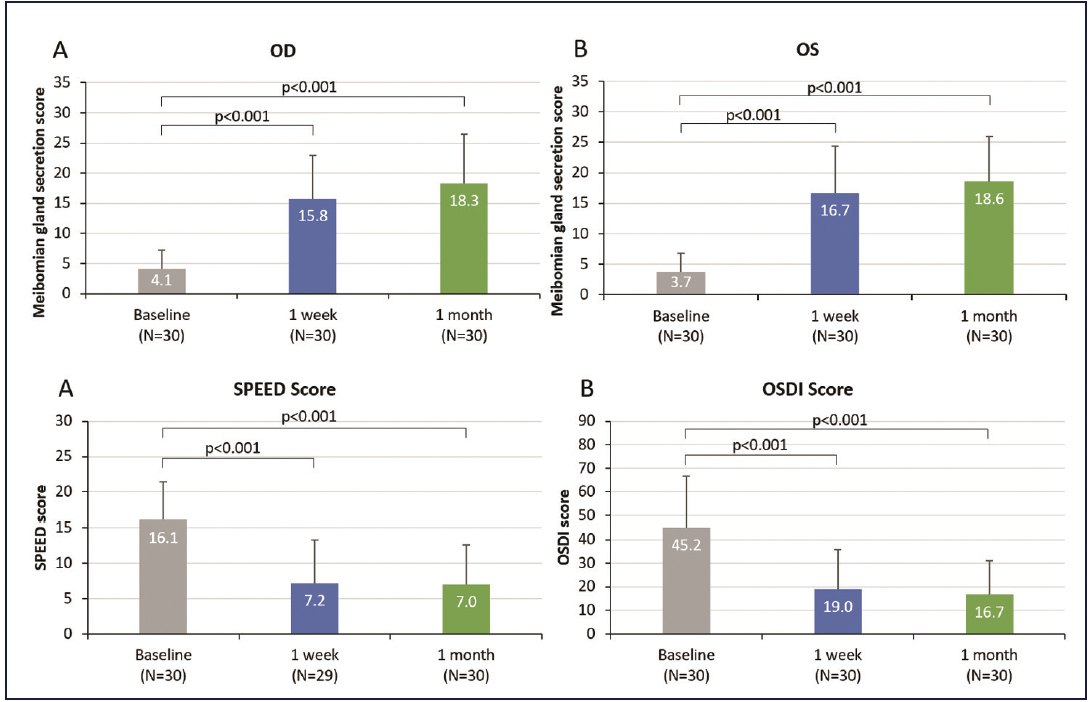
Figure 1. Systane iLux improved meibomian gland function and symptoms by one week after treatment.20
Dr. Gupta explained how the data impacts the patients. “When more healthy lipids are able to leave the glands, they spread on and protect the tear film and improve stability. An average MGS improvement of 11-13 means that 11-13 individual glands are each improving their meibum secretion by one unit. From a patient perspective, they are feeling better.6 From a clinician standpoint, not only are patients feeling better, but improved ocular surface protection allows for optimized pre-surgical measurements.7,8 How quickly this improvement happens makes this data very meaningful.” Dr. Devries concurred, “It’s incredible to be able to get your patients results and feeling better in such a short amount of time as 1 week. In just a week, you see significantly higher TBUT and dramatically improved symptoms because of the improved meibum flow from the glands.”
Not only does improvement come quickly, but it lasts. One month data are similar to 1 week data in the Schanzlin study, and a non-inferiority study that followed patients for 12 months after a single thermal pulsation treatment with iLux showed an average change of more than 16 units in MGS (Figure 2). “We want our patients to feel better quickly, but we also want therapy that lasts,” said Dr. Gupta. “What is really exciting is that you see a reduction in the average symptoms and increase average gland function similarly at 2 weeks and at 12 months.”
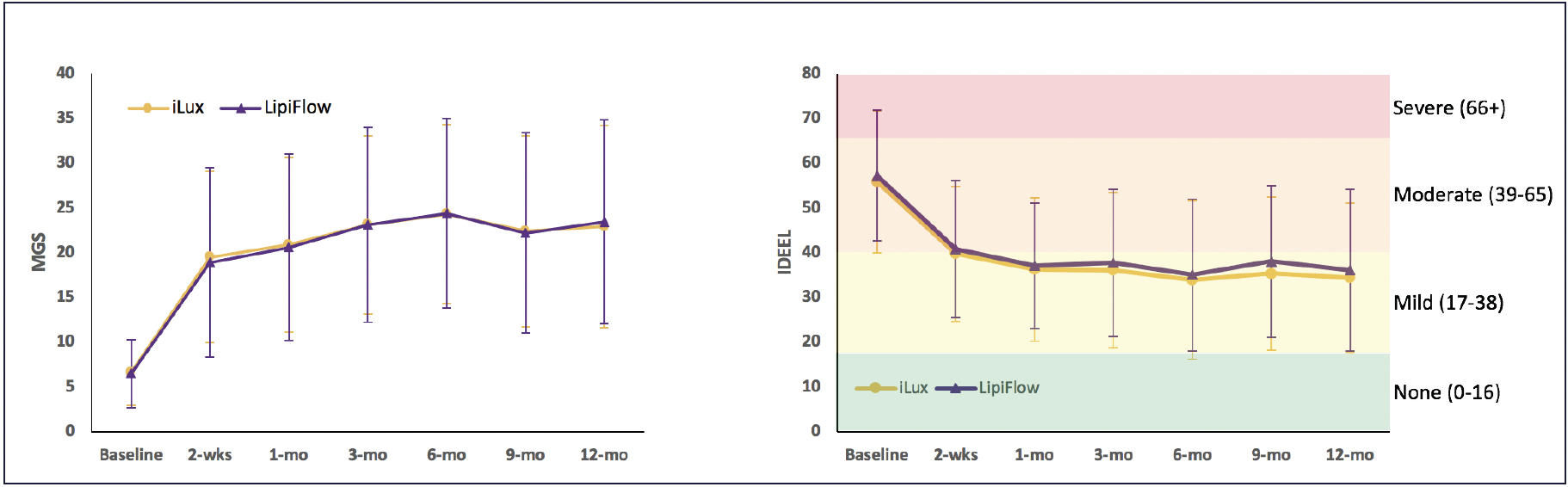
Figure 2. Results of a 12-month non-inferiority study comparing efficacy of Systane iLux to TearScience LipiFlow Thermal Pulsation System (Johnson & Johnson Vision). For both study groups, mean meibomian gland secretion score changed by >12 units by 2 weeks and >16 units by 12 months and mean IDEEL-SB was >55 units at baseline and <37 units at 12 months.21
Systane iLux2
There is now an upgraded version of the thermal pulsation system available, the Systane iLux2 (Figures 3 and 4). The smart tip, patient interface, heating, and compression mechanisms are the same, but additional features include a camera and real-time high resolution video display, as well as a recording system that captures white light and infrared images, including those of meibomian glands (Figure 5). “This may sound unnecessary, but the imaging is unique and really allows me to visually inspect the glands simultaneous to the expression,” reported Dr. Gupta. “That feedback is so important in allowing me to assess whether more heat and expression are needed in a specific area. In addition, the camera is a huge tool for patient education (Figure 6). When I can show them an image of me expressing a gland, the whole anatomy and disease state becomes very easy for the patient to grasp and adds another layer of credibility to the process. This is particularly valuable in patients with mismatching signs and symptoms.”
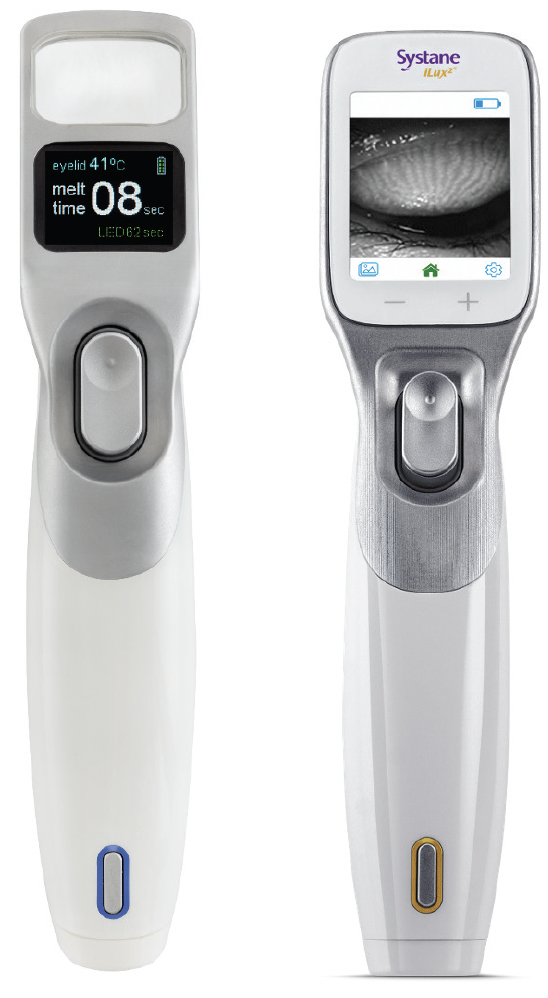
Figure 3. Systane iLux2 (right) replaces the magnifying window of the Systane iLux 1.5 (left) with a high-definition digital display.
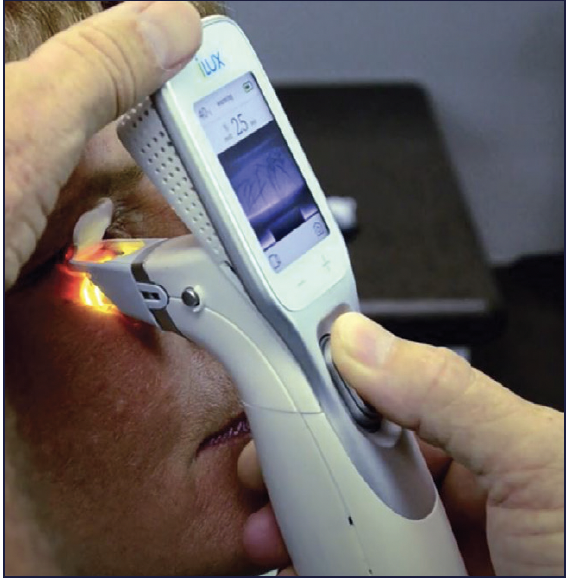
Figure 4. Systane iLux2 still allows visualization of the meibomian glands during treatment.
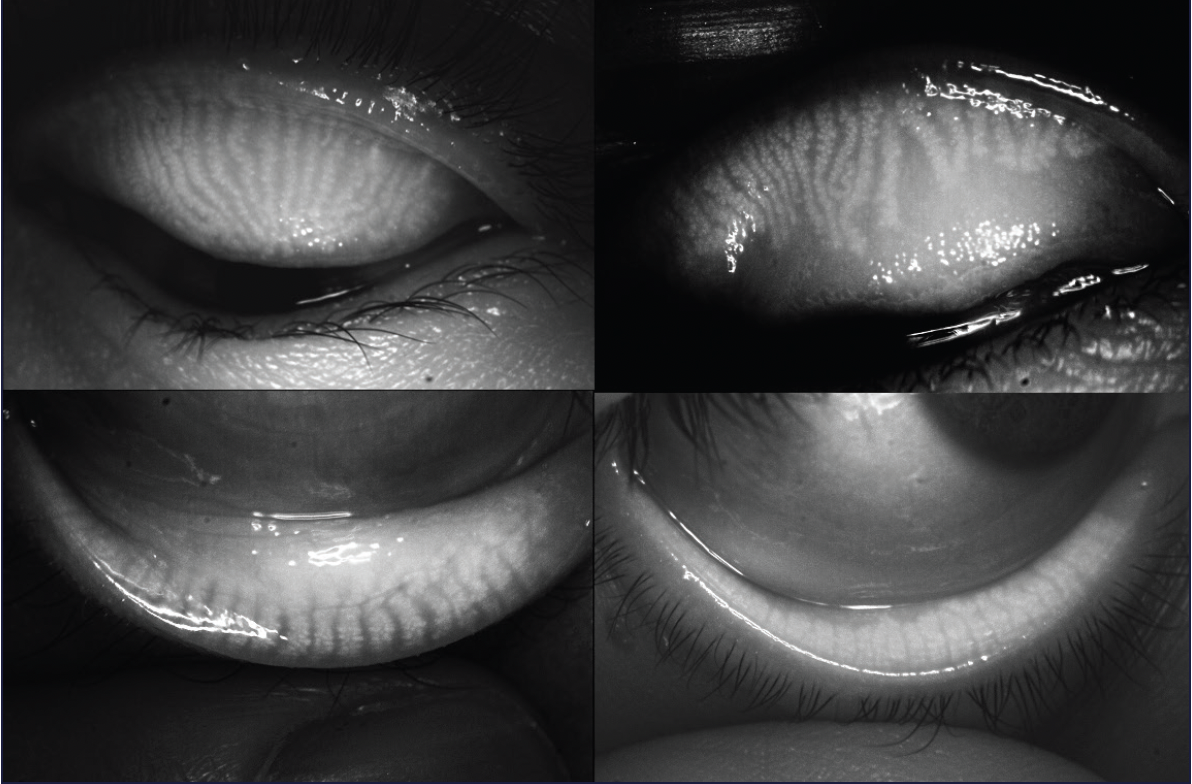
Figure 5. Systane iLux2 allows visualization, capture, and storage of meibomian gland images of both the upper and lower eyelids.
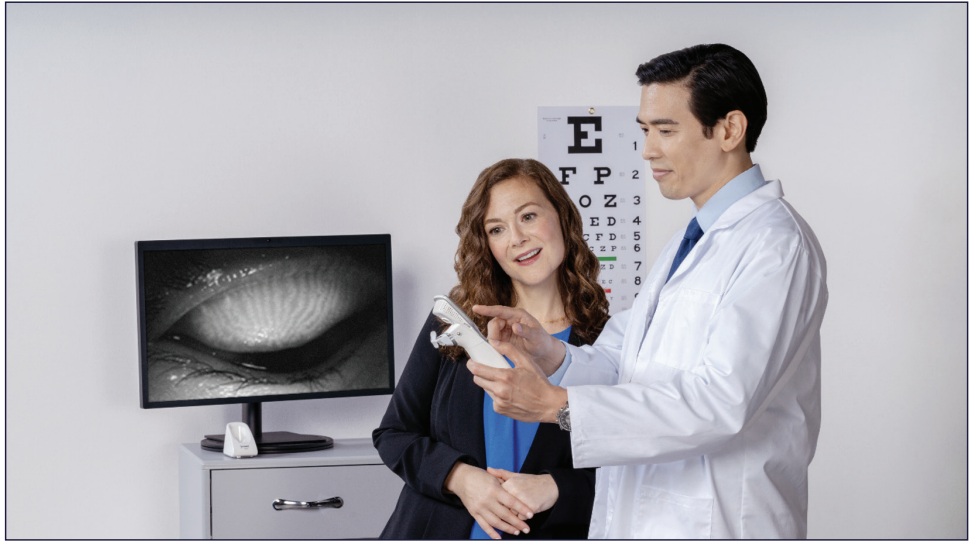
Figure 6. Images and videos captured and stored on Systane iLux2 can be viewed on the device or transferred to a hard drive for patient education and monitoring.
The panelists all concluded that the Systane iLux2 is not only a fast way to improve meibomian gland function and patient symptoms, but also an incredible education tool for patients. “The Systane iLux2 device is really bringing a high level of technology that is very easy to use,” stated Dr. Gupta. “That will drive practices to identify and treat a disease state that greatly impacts their patients.”
IMPORTANT PRODUCT INFORMATION
Indication: The Systane® iLux2® is indicated for the application of localized heat and pressure therapy in adult patients with meibomian gland dysfunction (MGD), which is associated with evaporative dry eye, and to capture/store digital images and video of the meibomian glands.
Contraindications:
Do NOT use the Systane® iLux2® in patients with the following conditions: Patients whose pupils have been pharmaceutically dilated; patients with ocular injury or trauma, chemical burns, or limbal stem cell deficiency (within prior 3 months); patients with active ocular herpes zoster or simplex of eye or eyelid or a history of these within prior 3 months; patients with cicatricial lid margin disease; patients with active ocular infection, active ocular inflammation or history of chronic, recurrent ocular inflammation within prior 3 months; patients with an ocular surface abnormality that may compromise corneal integrity; patients with lid surface abnormalities that affect lid function in either eye; patients with aphakia; or patients with permanent makeup or tattoos on their eyelids.
Warnings/Precautions:
Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner.
The Disposable may not fit all eyes, such as eyes with small palpebral fornices. Use of the Systane® iLux2® is NOT recommended in patients with the following conditions: moderate to severe allergic, vernal or giant papillary conjunctivitis; severe eyelid inflammation; systemic disease conditions that cause dry eye; in patients who are taking medications known to cause dryness; patients with punctal plugs, or patients who have undergone ocular surgery within prior 3 months.
Potential Adverse Reactions:
Potential adverse effects may occur because of the procedure. These effects include but are not limited to, the onset or increase in: eyelid/eye pain requiring discontinuation of the treatment procedure, eyelid irritation or inflammation, temporary reddening of the skin, ocular surface irritation or inflammation (e.g., corneal abrasion, conjunctival edema or conjunctival injection (hyperemia)), and ocular symptoms (e.g., burning, stinging, tearing, itching, discharge, redness, foreign body sensation, visual disturbance, sensitivity to light).
Attention: Please refer to the User Manual for a complete list of contraindications, instructions for use, warnings and precautions for the Systane® iLux2®.
US-IL2-2200004
1. TFOS. What Is DEWS II. Available at http://www.tearfilm.org/dettreports-tfos_dew_ii_report/32_30/eng/. Accessed May 9, 2018.
2. Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90-98.
3. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478.
4. Cochener B, Cassan A, Omiel L. Prevalence of meibomian gland dysfunction at the time of cataract surgery. J Cataract Refract Surg. 2018;44(2):144-148.
5. Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O’Brien T, Rolando M, Tsubota K, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050-2064.
6. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27(20):1142-1147.
7. Epitropoulos AT, Matossian C, Berdy GJ, Malhotra RP, Potvin R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41(8):1672-1677.
8. Matossian C. Impact of thermal pulsation treatment on astigmatism management and outcomes in meibomian gland dysfunction patients undergoing cataract surgery. Clin Ophthalmol. 2020;14:2283-2289.
9. 2007 Report of the International Dry Eye Workshop (DEWS). The Ocular Surface. 2007;5:65-204. For a full copy of the DEWS report, please visit the TFOS website: www.tfos.org.
10. Koh S, Tung CI, Inoue Y, Jhanji V. Effects of tear film dynamics on quality of vision. Br J Ophthalmol. 2018;102(12):1615-1620.
11. Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52(4):1994-2005.
12. Borchman D, Ramasubramanian A, Foulks GN. Human meibum cholesteryl and wax ester variability with age, sex, and meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2019;60(6):2286-2293.
13. Shine WE, McCulley JP. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch Ophthalmol. 1998;116(7):849-852.
14. Borchman D, Foulks GN, Yappert MC, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(6):3805-3817.
15. Alghamdi YA, Camp A, Feuer W, Karp CL, Wellik S, Galor A. Compliance and subjective patient responses to eyelid hygiene. Eye Contact Lens. 2017;43(4):213.
16. Lane SS, DuBiner HB, Epstein RJ, Ernest PH, Greiner JV, Hardten DR, Holland EJ, Lemp MA, McDonald JE, Silbert DI, Blackie CA. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31(4):396-404.
17. Calderhead, R. G., & Tanaka, Y. (2017). Photobiological Basics and Clinical Indications of Phototherapy for Skin Rejuvenation. In Photomedicine – Advances in Clinical Practice. InTech. https://doi.org/10.5772/intechopen.68723
18. Arita R, Shirakawa R, Maeda S, et al. Decreased surface temperature of tarsal conjunctiva in patients with meibomian gland dysfunction. JAMA Ophthalmol. 2013;131(6):818-819.
19. Borchman D. The optimum temperature for the heat therapy for meibomian gland dysfunction. Ocul Surf. 2019;17(2):360-364.
20. Schanzlin D, Owen JP, Klein S, Yeh TN, Merchea MM, Bullimore MA. Efficacy of the systane iLux thermal pulsation system for the treatment of meibomian gland dysfunction after 1 week and 1 month: a prospective study. Eye & Contact Lens. 2021.
21. Alcon Data on File.






