When I first started offering the AcrySof IQ ReSTOR +2.5 D IOL with ACTIVEFOCUS optical design (Alcon), I assumed it would be great for uncomplicated cases—patients with a healthy ocular surface and no macula or retina pathology. The lens has certainly met my expectations in this regard, but I have also been pleasantly surprised with how well it has performed for traditionally challenging patients, like those with mildly altered corneas due to prior refractive surgery, and those who have mild dry eye or who have had prior dry eye treatment before surgery.
With a central zone targeted to distance vision, the AcrySof IQ ReSTOR +2.5 D IOL with ACTIVEFOCUS optical design is almost like using a monofocal IOL (Figure).1-5 In my experience, it is more forgiving of residual refractive error,6 and more often than not, it provides the full range of vision that patients want after opting for a multifocal IOL.
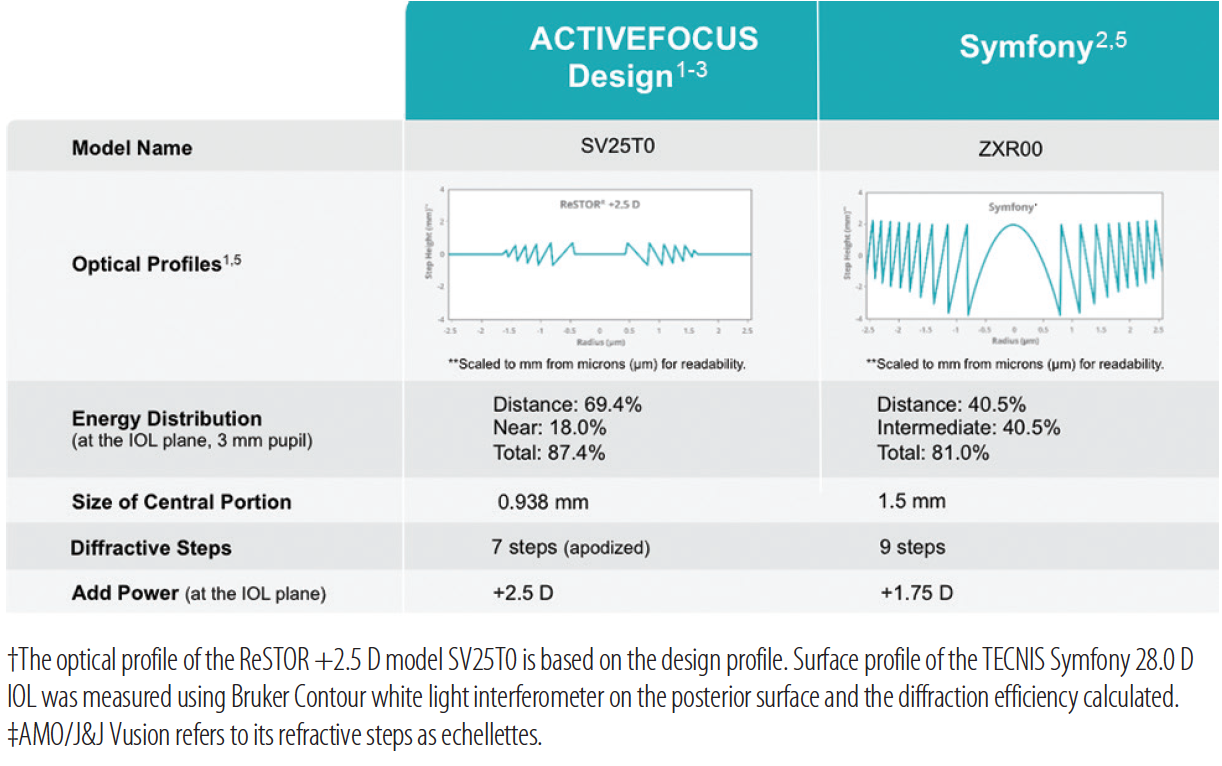
Figure. Comparison of key features of presbyopia-correcting IOLs.1-5
Yet, while many patients will succeed with bilateral implantation of this IOL targeting plano and emmetropia, for some patients, a little bit extra near vision is needed for activities and hobbies. In these settings, the family of AcrySof IQ ReSTOR lenses provides a few different options to help patients achieve their vision goals.
We have studied outcomes with contralateral implantation and mini-monovision in two separate studies.7 In the first study, a group of patients received the AcrySof IQ ReSTOR +2.5 D IOL with ACTIVEFOCUS optical design in the dominant eye and the AcrySof IQ ReSTOR +3.0 D IOL in the second eye. We found that patients were very satisfied with their outcome and experienced fewer glares and halos than patients who received an AcrySof IQ ReSTOR +3.0 D IOL in each eye.
In the second study, which is currently ongoing, we are implanting the AcrySof IQ ReSTOR +2.5 D IOL with ACTIVEFOCUS optical design bilaterally but aiming for 0.5 D myopia in the nondominant eye. Our early results with this approach have been very positive (unpublished data).
Framing the conversation about postoperative vision around practical examples has helped educate about outcomes and manage expectations. The perfect IOL that gives patients excellent distance, great near vision, and everything in between, simply does not exist. In my view, the most promising option we have to provide uncompromised distance with a full range of vision is the AcrySof IQ ReSTOR +2.5 D IOL with ACTIVEFOCUS optical design.
I tell patients that if I put this lens in each eye, they will have a good chance of achieving vision that will help them drive, play golf, read road signs, and see the dashboard or computer screen, but they may need glasses to read fine print up close. I then offer them the option of making one eye “not as good for distance, but it will be better in the near range.” I have found that is usually all the explanation that patients need or want.
We implant a lot of premium IOLs in our practice—around 80% to 90% of cataract patients choose some type of upgrade. We are excited about the various multifocal, presbyopia-correcting, and toric platforms that are available, because they provide patients with numerous options to achieve the vision they want.
We offer the AcrySof IQ ReSTOR +2.5 D IOL with ACTIVEFOCUS optical design to appropriate candidates because it is a great option to achieve vision from distance to about arm’s length. And for those patients who want a little extra near vision, the potential to aim for mini-monovision or to perform contralateral implantation with an AcrySof IQ ReSTOR +3.0 D IOL allows us to truly customize the approach to vision correction.8
1. Alcon Data on File (April 11, 2016).
2. Alcon Data on File (Oct. 17, 2016).
3. AcrySof IQ ReSTOR +2.5 D IOL Directions for Use.
4. Alcon Data on File (Aug. 7, 2013).
5. TECNIS Symfony Extended Range of Vision IOL Directions for Use.
6. Alcon Data on File (Oct. 6, 2016).
7. Hovanesian JA. Patient-reported satisfaction and spectacle independence with the 2.5 D multifocal IOL combined with the 3.0 D model in cataract surgery versus bilateral implantation of the 3.0 D model. Presented at: ASCRS Annual Meeting; April 12-16, 2018; Washington, DC.
8. AcrySof IQ ReSTOR +3.0 D IOL Directions for Use.
AcrySof, ACTIVEFOCUS and ReSTOR are trademarks of Novartis. All other brand/product names are the trademarks of their respective owners.
©2019 Novartis 01/19 US-RES-18-E-2609a
AcrySof IQ ReSTOR Family of Multifocal IOLs Important Product Information
CAUTION: Federal law restricts these devices to sale by or on the order of a physician.
INDICATION: The family of AcrySof® single-piece intraocular lenses (IOLs) includes AcrySof® UV-absorbing IOLs (“AcrySof® UV”), AcrySof ®IQ, AcrySof ®IQ Toric® and AcrySof IQ ReSTOR® and AcrySof® IQ ReSTOR® Toric IOLs. Each of these IOLs is indicated for visual correction of aphakia in adult patients following cataract surgery. In addition, the AcrySof Toric IOLs are indicated to correct pre-existing corneal astigmatism at the time of cataract surgery. The AcrySof IQ ReSTOR IOLs are for cataract patients with or without presbyopia, who desire increased spectacle independence with a multifocal vision. All of these IOLs are intended for placement in the capsular bag.
WARNINGS/PRECAUTIONS:
General cautions for all AcrySof® and AcrySof® UV IOLs:
Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting any IOL in a patient with any of the conditions described in the Directions for Use that accompany each IOL. Caution should be used prior to lens encapsulation to avoid lens decentration or dislocation. Viscoelastic should be removed from the eye at the close of surgery.
Additional Cautions associated with AcrySof® IQ ReSTOR® IOLs: Some patients may experience visual disturbances and/or discomfort due to multifocality, especially under dim light conditions. A reduction in contrast sensitivity may occur in low light conditions. Visual symptoms may be significant enough that the patient will request explant of the multifocal IOL. Spectacle independence rates vary with all multifocal IOLs; as such, some patients may need glasses when reading small print or looking at small objects. Clinical studies indicate that posterior capsule opacification (PCO), when present, may develop earlier into clinically significant PCO with multifocal IOLs.
Additional Cautions associated with AcrySof® IQ Toric, AcrySof® UV Toric and ReSTOR® Toric IOLs: Optical theory suggests that, high astigmatic patients (i.e. > 2.5 D) may experience spatial distortions. Possible toric IOL related factors may include residual cylindrical error or axis misalignments. Toric IOLs should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation.
Prior to surgery, physicians should provide prospective patients with a copy of the appropriate Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with the AcrySof® IQ Toric, AcrySof® IQ ReSTOR® and AcrySof® IQ ReSTOR® Toric IOLs.
Do not resterilize. Do not store at temperatures over 45° C. Use only sterile irrigating solutions to rinse or soak IOLs.
ATTENTION: Refer to the Directions for Use labeling for the specific IOL for a complete list of indications, warnings and precautions.

