Bennett Walton, MD
Bennett Walton Vision, Houston
At Slade & Baker Vision in Houston, our cataract patients are definitely refractive patients. Even for those who present with some sort of surgical challenge, such as surface dryness, Fuchs dystrophy, a dense cataract, or history of ocular trauma, we recognize that they still want a good refractive result.
We can exceed patients’ expectations in two primary ways. First, we can prepare them for the postoperative experience we expect they’ll have based on the preoperative examination. Second, we can perform the most skillful surgery possible, which includes using the best instruments and tools. One of my most reliable surgical tools is the DuoVisc Viscoelastic System (Alcon Vision LLC), which I use in almost all my surgeries. The DuoVisc package contains the dispersive viscoelastic Viscoat® OVD, which I find coats the cornea effectively and protects it well.1-4 It is the only dispersive OVD that contains 4% chondroitin sulfate for strong adherence to the corneal endothelium.5-7 In DuoVisc, Viscoat is paired with the cohesive Provisc® OVD, which excels at creating space in the capsular bag due to its high molecular weight.8 Yet, Provisc provides excellent clarity and is also easy to remove at the end of surgery.
Protecting endothelial cells and supporting the capsular bag are two separate surgical functions that require two different viscoelastic tools for optimal performance. DuoVisc has both of these tools to ensure that I get the results I expect.1,2,6,8,9
Case Example: Fuchs Dystrophy
Recently, a 63-year-old female presented to me complaining of difficulty driving at night with her right eye. I diagnosed the eye with Fuchs’ dystrophy plus cataract and a preoperative BCVA of 20/40. I estimated that 70% of the visual limitation was from the cataract, and 30% was from the corneal pathology. Because of the Fuchs dystrophy, I counseled this patient to expect a slow healing response and a delayed return to functional vision. The Fuchs dystrophy also limited the IOL options we could recommend, but still, she wanted to get the best vision she could.
Technique
I begin all cases by applying epi-Shugarcaine solution (epinephrine 0.025% and lidocaine 0.75% in BSS Plus solution) for intracameral dilation. Then, I fill the eye distally to proximally with Viscoat, so that all the structures are nicely coated. In this eye, Viscoat provided stabilization while I removed the cataract.
When I use the femtosecond laser, I use three steps in Viscoat injection: stabilize the capsulotomy; evacuate bubbles; and viscodilate the pupil (Figure 1). If there’s an adhesion in the capsulotomy that needs to be released with forceps, I will add more Viscoat to the chamber, because it does such a great job of stabilizing all structures and giving me space to complete the capsulotomy. When I want to stabilize the eye, I think about whether I want to divide regions of the eye or just fill them. I usually prefer Viscoat for making a wall and dividing space within the eye, and I use Provisc to hold open spaces, primarily the capsular bag. Also, Provisc is easily removable at the end of surgery.8
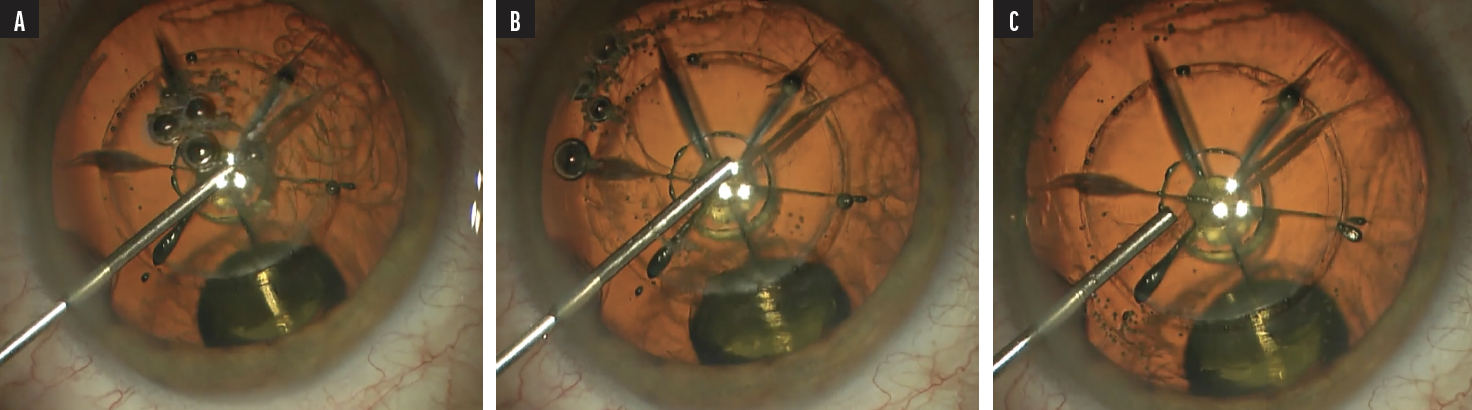
Figure 1. When initiating a femtosecond cataract case, Dr. Walton first places Viscoat on top of the capsulotomy (A) to stabilize it prior to inspection for any tags. With continued injection of Viscoat from distal to proximal (B), bubbles are self-evacuating toward the incision for best vision, and then the iris may be held down and open with the completion of Viscoat injection (C).
With the cataract out, I removed the cortex and filled the capsular bag with an ample amount of Provisc.
Once the IOL is in place, I like to angle the aspirator tip underneath the IOL to remove the Provisc, especially when I am implanting toric IOLs. This approach evacuates all remaining viscoelastic and reduces the chance of an IOP spike. I look for swirls of Provisc both behind the IOL and in the anterior chamber as it exits. Extracting the viscoelastic usually takes me around 10 seconds. It comes out very easily with the right balance of inflow and outflow settings. After removing the Provisc centrally, I maneuver the aspirator around the anterior chamber to remove any residual Viscoat.
Dr. Walton’s Pearls for Using DuoVisc
- If you think there could be a nuclear fragment hidden in the sulcus behind the iris, a little bit of Provisc injected and swept around can be very helpful.
- With dense cataracts, I use extra Viscoat to re-coat the endothelium and protect it. I believe this step provides the protection that these patients need.
- In certain complex cases where there may be an upward pressure of silicon oil in the back of the eye, filling the OVD from a paracentesis incision may prevent the pressure from the silicon oil from pushing the viscoelastic right back out of the main incision.
- For a capsule rupture, use Viscoat through a paracentesis with the nondominant hand to stabilize before coming out with the irrigation. The goals are both to avoid a negative flow gradient out the wound when withdrawing the phaco handpiece, and to create a wall against more vitreous coming forward through the capsular rent.
Despite her Fuchs diagnosis, this patient was able to achieve 20/25+ with only mild corneal edema on day 1. That wow effect was huge for her, and this outcome allowed her to delay a DMEK procedure.
The Importance of Endothelial Adherence in an OVD
I believe that fluidics has become a bigger influence on corneal clarity following typical cataract cases at day 1 than the ultrasound itself with modern phaco machines. I used to associate day-1 corneal edema with excessive ultrasound, but I’ve seen enough postops from other non-ultrasound procedures that now I think fluid flow over the endothelium is equally, if not more important. Viscoat buffers the endothelium from experiencing stress from that fluid flow—it is important to have the protective properties of Viscoat, with the inclusion of chondroitin sulfate, to prevent the undue loss of endothelial cells. Several studies have shown that OVDs that contain chondroitin sulfate keep the endothelium coated during high-flow fluidics.1-4,6,7
DuoVisc Included With the Alcon Custom Pak®
DuoVisc Viscoelastic System is available as a standalone agent or as part of the Custom Pak® surgical procedure pack that surgeons may order from Alcon. These comprehensive surgical packs for cataract and retinal surgeries can be customized to the surgeon’s exact specifications and are organized to maximize efficiency and minimize error in the OR. Contact your local sales representative or visit the My Alcon Store to see all options and to order online. As the only all-in-one ophthalmic surgical procedure pack, the Alcon Custom Pak® offers the following:
- Best-in-class tools as well as everyday staples from trusted suppliers, all sterilized and configured in your preferred sequence
- Only all-in-one surgical kit that can be ordered with one phone call and paid for with one invoice.
- Personalized service
- A reliable kit that streamlines surgical prep
- Bundling items, which saves costs
- End-to-end ordering, billing, and shipping, which saves time
Alison Early, MD
Cincinnati Eye Institute, Ohio
I am a cataract and refractive surgeon at the Cincinnati Eye Institute. At my primary location in a far northern area between Cincinnati and Dayton, I see a lot of pathology and many hypermature dense cataracts. Throughout my years of training and now in practice, I have had great success with the Alcon surgical platform as well as DuoVisc, my OVD of choice. With DuoVisc, I achieve consistent surgical results with great corneal recovery,1,6 and I continue to confidently use the two viscoelastics in DuoVisc: Viscoat (dispersive) and Provisc (cohesive).
DuoVisc gives me fantastic visualization during surgery, and I find the quantity in the syringes to be adequate to achieve the right pressurization of the globe and of the capsular bag when I’m getting ready to implant a lens. When I am operating on large eyes, high myopes, or performing a MIGS procedure, DuoVisc gives me perfect visualization of the angle using the gonio prism.
Technique
In a typical surgery, I make the paracentesis incision, then I instill some intracameral anesthetic. Next, I fill the anterior chamber with Viscoat to maintain its shape and pressurization. I think Viscoat does a fantastic job. It stays in the eye because of its triple negative charge, which comes from its combined solution of chondroitin sulfate (a double-negative charge) and sodium hyaluronate (a single negative charge). The corneal endothelium’s positive charge creates a molecular attraction5 that enables Viscoat to adhere to it. The way that Viscoat reliably adheres to the corneal endothelium gives me reassurance that the eye is protected during surgery, particularly in cases where there may be baseline endothelial dystrophy.
Dr. Early’s Pearls for Using DuoVisc
- Use Arshinoff’s soft-shell technique for endothelial protection: first partially fill the anterior chamber with Viscoat, then instill Provisc underneath.10
- Pressurizing the AC with Viscoat prior to puncturing the anterior capsule of an intumescent cataract is integral to creating a successful continuous curvilinear capsulorhexis and avoiding the Argentinean flag complication.
- DuoVisc provides pressurization and visualization of the anterior chamber angle for MIGS procedures such as Hydrus Microstent implantation.
- Thorough evacuation of OVD from the anterior chamber angle as well as from behind the IOL helps to avoid postoperative IOP spikes.
For very dense cataracts in which I anticipate using high phaco energy, I favor the soft-shell technique that was published by Steve Arshinoff.10 I instill the dispersive Viscoat first, and then underneath that, I add a bolus of cohesive Provisc viscoelastic. I find this technique to be incredibly protective of the corneal endothelium; I see very clear corneas on postoperative day 1.
The “Brown Bear” Cataract
Last year, I operated on the brownest, densest cataract I’ve ever seen. I called this cataract the “Brown Bear,” because it was the color of cola. This patient was a 69-year-old male with a history of heavy smoking. Preoperatively, his BCVA was 20/400 with a reddish-brown nuclear cataract.
For this case, I anticipated that the CDE would be very high, which can lead to significant postoperative corneal edema and slower clearing of vision if the corneal endothelium is not adequately protected intraoperatively. After anesthetizing the eye with a peribulbar block, I applied the soft-shell technique. This is the most effective way to ensure protection of the corneal endothelium. Because the red reflex was quite poor, I opted for an out-of-the-bag chop technique. I used Viscoat to prolapse the nucleus out of the capsular bag for disassembly (Figure 2); Viscoat also occupied the space between the posterior capsule and the lens during this step. I told the scrub tech to be ready for anything, because I could not see what was going on with the capsule until I got the first nuclear fragments out.
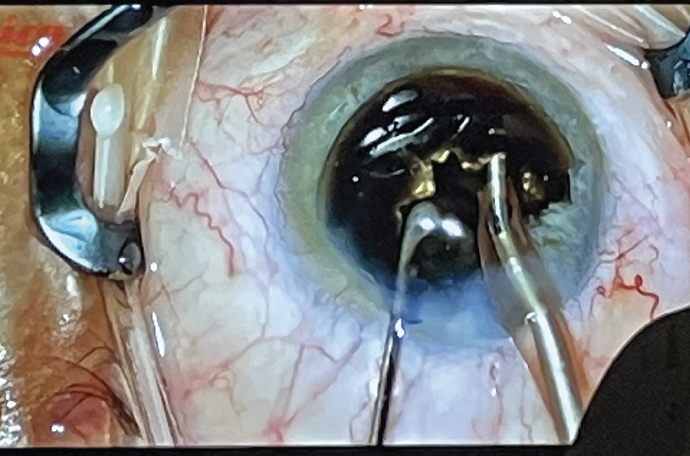
Figure 2. Viscoat maintained the space between the posterior capsule and the lens while Dr. Early disassembled the brown nucleus using an out-of-the-bag chop technique.
I was able to successfully remove this lens in approximately 10 minutes. The capsular bag was perfectly intact, and I was able to implant an Alcon Clareon® single-piece monofocal IOL in the bag.
Within a few hours after surgery, this patient was already seeing 20/100, and by 1 week, his UVCA was 20/30. I am sure that the soft-shell technique using DuoVisc facilitated this patient’s quick recovery.
DuoVisc in White Cataracts
The preparation and approach for handling a dense brunescent cataract is quite different from the approach to an hypermature, intumescent white cataract. For the latter, I also heavily depend on the properties of DuoVisc and have developed a reliable technique. First, I use Viscoat to fully fill and pressurize the anterior chamber after staining the anterior capsule with trypan blue dye. Maintaining a high pressure in the anterior chamber protects against the dreaded Argentinean flag, which occurs after abrupt depressurization of the capsular bag. I then enter the eye with a 27-gauge needle on a 3-cc syringe partially filled with BSS. Immediately after puncturing the anterior capsule, I draw back on the syringe to aspirate lens material and depressurize the capsular tension. This technique is successful when the anterior chamber is adequately pressurized with viscoelastic, so that there is not a significant pressure gradient or differential between it and the bag.
Often, white lenses will also have a thin, friable posterior capsule, since it has been under tremendous tension prior to surgery. Continually filling with Viscoat to maintain the capsule space during the rest of nuclear removal and cortical cleanup allows the surgeon to successfully tame the “great white shark” cataract.
I love using Viscoat to maintain the capsular space in any surgery where I’m concerned about the capsular bag becoming floppy. If the bag looks unstable, I instill some Viscoat after extracting perhaps half of the nuclear fragments. Provisc excels at keeping the bag inflated after the removal of all lens material, and then it is easy to remove in one fell swoop once the IOL is in place.
Case Example: High Myope With Previous RK
Recently, I performed cataract surgery on a patient who had previously undergone radial keratotomy (RK) for high myopia. She now had a cataract and moderate open-angle glaucoma in both eyes. I planned to perform a combined cataract surgery with phacoemulsification and microinvasive glaucoma surgery (MIGS) with a Hydrus® Microstent in each eye separately.
I made the incisions, filled the chamber with Viscoat, phacoemulsified the nucleus, and implanted the IOL. In large, highly myopic eyes, such as this patient’s, the anterior chamber becomes incredibly deep following cataract extraction. I palpated with the cannula and found that the anterior chamber of this eye was not firm, so I opened a second syringe of Viscoat and refilled the chamber until it was firm enough to allow beautiful visualization of the angle for the Hydrus® Microstent.
I coated my gonioprism with OVD and placed it on the cornea. The technique requires a delicate balance of applying enough pressure on the cornea to visualize the angle and stabilize the eye, but not enough pressure to flatten or cause folds in the cornea, which can impede visualization. I find that using Viscoat in the anterior chamber throughout the procedure keeps the eye nicely pressurized. I have no issues with the OVD escaping out the incisions when I put light pressure on the cornea (Figure 3).
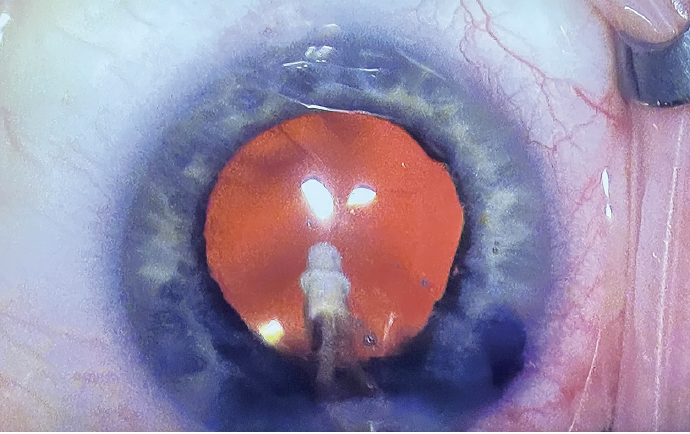
Figure 3. The conjunctiva blanched nicely after Dr. Early implanted the Hydrus Microstent in the angle of an eye that received simultaneous cataract surgery. Dr. Early prefers to use Viscoat in the capsular angle for its excellent clarity during MIGS procedures.
With the viscoelastic in place, I could perform Hydrus Microstent implantation with a clear view. I feel that the clarity of Viscoat is good for angle visualization during MIGS. I entered Schlemm’s canal and smoothly deployed the stent. I could make minor adjustments to the position of the microstent with a second instrument—which meant entering and exiting the eye a few times—without losing the pressurization of the globe due to the viscoelastic.
AVOIDING IOP SPIKES
Using DuoVisc, I almost never see postoperative IOP spikes in routine cases. One technique that I have shared with other surgeons is to avoid aggressively injecting dispersive OVD far into the angle at the beginning of a cataract surgery. I start administering OVD at the edge of the dilated iris, and I focus on filing the central region of the anterior chamber. At the end of a case when I am removing the OVD, I sweep around the angle with my I/A cannula to make sure no OVD is left behind. It is also important to remove any residual OVD from behind the IOL before the case is complete to avoid issues with IOP as well as refractive surprises due to trapped OVD in rare cases.
In summary, I find DuoVisc to be reliable in planned procedures and versatile for unexpected situations. I always feel confident when I have DuoVisc on my tray.
Important Product Information for DUOVISC® OVD
Description: DUOVISC® Viscoelastic System is designed to provide two Viscoelastic materials with different physico-chemical properties that can be used differently and/or sequentially to perform specific tasks during a cataract procedure. DUOVISC® Viscoelastic System consists of VISCOAT® Ophthalmic Viscosurgical Device and PROVISC® Ophthalmic Viscosurgical Device.
Caution: Federal (USA) law restricts this device to sale by, or on the order of, a physician.
Description: VISCOAT® (Sodium Chondroitin Sulfate – Sodium Hyaluronate) Ophthalmic Viscosurgical Device
Indications: VISCOAT® OVD is indicated for use as an ophthalmic surgical aid in anterior segment procedures including cataract extraction and intraocular lens (IOL) implantation. VISCOAT® OVD maintains a deep anterior chamber during anterior segment surgeries, enhances visualization during the surgical procedure, and protects the corneal endothelium and other ocular tissues. The viscoelasticity of the solution maintains the normal position of the vitreous face and prevents formation of a flat chamber during surgery.
Warnings/Precautions: Failure to follow assembly instructions or use of an alternate cannula may result in cannula detachment and potential patient injury. Precautions are limited to those normally associated with the surgical procedure being performed. Although sodium hyaluronate and sodium chondroitin sulfate are highly purified biological polymers, the physician should be aware of the potential allergic risks inherent in the use of any biological material.
Adverse Reactions: VISCOAT® OVD has been extremely well tolerated in human and animal studies. A transient rise in intraocular pressure in the early postoperative period may be expected due to the presence of sodium hyaluronate, which has been shown to affect such a rise. It is therefore recommended that VISCOAT® OVD be removed from the anterior chamber by thorough irrigation and/or aspiration at the end of surgery to minimize postoperative IOP increases. Do not overfill anterior chamber.
ATTENTION: Please refer to the Directions for Use for a complete listing of indications, warnings and precautions.
Description: PROVISC® (Sodium Hyaluronate) Ophthalmic Viscosurgical Device
Indications: PROVISC® OVD is indicated for use as an ophthalmic surgical aid in the anterior segment during cataract extraction and intraocular lens (IOL) implantation. Ophthalmic viscoelastics serve to maintain a deep anterior chamber during anterior segment surgery allowing reduced trauma to the corneal endothelium and surrounding ocular tissues. They help push back the vitreous face and prevent formation of a flat chamber during surgery.
Warnings/Precautions: Postoperative increases in intraocular pressure have been reported with sodium hyaluronate products. The IOP should be carefully monitored and appropriate therapy instituted if significant increases should occur. It is recommended that PROVISC® OVD be removed by irrigation and/or aspiration at the close of surgery. Do not overfill anterior chamber. Although sodium hyaluronate is a highly purified biological polymer, the physician should be aware of the potential allergic risks inherent in the use of any biological material; care should be used in patients with hypersensitivity to any components in this material. Cannula assembly instructions should be followed to prevent patient injury.
Adverse Reactions: Postoperative inflammatory reactions such as hypopyon and iritis have been reported with the use of ophthalmic viscoelastics, as well as incidents of corneal edema, corneal decompensation, and a transient rise in intraocular pressure.
ATTENTION: Please refer to the directions for use for a complete listing of indications, warnings and precautions.
HYDRUS® MICROSTENT IMPORTANT SAFETY INFORMATION
INDICATIONS FOR USE: The Hydrus Microstent is indicated for the reduction of intraocular pressure (IOP) in adult patients with primary open angle glaucoma (POAG) as a standalone treatment or in conjunction with cataract surgery.
CONTRAINDICATIONS: The Hydrus Microstent is contraindicated under the following circumstances or conditions: (1) In eyes with angle closure glaucoma; and (2) In eyes with secondary glaucoma such as neovascular, uveitic, traumatic, or steroid-induced glaucoma, and glaucoma associated with increased episcleral venous pressure (EVP) e.g., Sturge-Weber Syndrome; (3) patients with known nickel allergy.
WARNINGS: Clear media for adequate visualization is required. Conditions such as corneal haze, corneal opacity or other conditions may inhibit gonioscopic view of the intended implant location. Gonioscopy should be performed prior to surgery to exclude congenital anomalies of the angle, peripheral anterior synechiae (PAS), angle closure, rubeosis and any other angle abnormalities that could lead to improper placement of the stent and pose a hazard. The surgeon should monitor the patient postoperatively for proper maintenance of intraocular pressure. The surgeon should periodically monitor the status of the microstent with gonioscopy to assess for the development of PAS, obstruction of the inlet, migration, or device-iris or device-cornea touch. The Hydrus Microstent is indicated for implantation in conjunction with cataract surgery, which may impact corneal health. Therefore, caution is indicated in eyes with evidence of corneal compromise or with risk factors for corneal compromise following cataract surgery. Prior to implantation, patients with history of allergic reactions to nitinol, nickel or titanium should be counseled on the materials contained in the device, as well as potential for allergy/hypersensitivity to these materials.
PRECAUTIONS: If excessive resistance is encountered during the insertion of the microstent at any time during the procedure, discontinue use of the device. The safety and effectiveness of use of more than a single Hydrus Microstent or with other metallic implants has not been established. The safety and effectiveness of the Hydrus Microstent has not been established as an alternative to the primary treatment of glaucoma with medications, in patients 21 years or younger, eyes with significant prior trauma, eyes with abnormal anterior segment, eyes with chronic inflammation, eyes with glaucoma associated with vascular disorders, eyes with preexisting pseudophakia, eyes with pseudoexfoliative or pigmentary glaucoma, and when implantation is without concomitant cataract surgery with IOL implantation. Please see a complete list of Precautions in the Instructions for use.
ADVERSE EVENTS: The most frequently reported finding in the randomized pivotal trial was peripheral anterior synechiae (PAS), with the cumulative rate at 5 years (14.6% vs 3.7% for cataract surgery alone). Other Hydrus postoperative adverse events reported at 5 years included partial or complete device obstruction (8.4%) and device malposition (1.4%). Additionally, there were no new reports of persistent anterior uveitis (2/369, 0.5% at 2 years) from 2 to 5 years postoperative. There were no reports of explanted Hydrus implants over the 5-year follow-up. For additional adverse event information, please refer to the Instructions for Use.
MRI INFORMATION: The Hydrus Microstent is MR-Conditional meaning that the device is safe for use in a specified MR environment under specified conditions.
Please see the Instructions for Use for complete product information
© Alcon Inc. 12/23 US-DUV-2300010
1. Glasser DB, Katz HR, Boyd JE, Shobe SL, Peiffer RL. Protective effects of viscous solutions in phacoemulsification and traumatic lens implantation. Arch Ophthalmol.1989;107(7):1047-1051.
2. Papaconstantinou D, Karmiris T, Diagourtas A, Koutsandrea C, Georgalas I. Clinical trial evaluating Viscoat® and Visthesia ophthalmic viscosurgical devices in corneal endothelial loss after cataract extraction and intraocular lens implantation. Cutan Ocul Toxicol. 2014;33(3):173-180.
3. Koch D. et al. A comparison of corneal endothelial changes after use of Healon or Viscoat during phacoemulsification. Am J Ophthalmol. 1993;115(2):188-201.
4. Moschos MM, Chatziralli IP, Sergentanis TN. Viscoat versus Visthesia during phacoemulsification cataract surgery: corneal and foveal changes. BMC Ophthalmol. 2011;11:9.
5. Hsaio CW, Cheng H, Ghafouri R, Ferko NC, Ayres BD. Corneal outcomes following cataract surgery using ophthalmic viscosurgical devices composed of chondroitin sulfate-hyaluronic acid: a systematic review and meta-analysis. Clin Ophthalmol. 2023; 17: 2083–2096.
6. Petroll WM, Jafari M, Lane SS, Jester JV, Cavanagh HD. Quantitative assessment of ophthalmic viscosurgical retention using in vivo confocal microscopy. J Cataract Refract Surg. 2005;31(12):2363-2368.
7. Poyer JF, Chan KY, Arshinoff SA. New method to measure the retention of viscoelastic agents on rabbit corneal endothelial cell line after irrigation and aspiration. J Cataract Refract Surg. 1998;24(1):84-90.
8. DisCoVisc® OVD Product Insert.
9. Lindstrom RL, Ong M. Protective effect of OVDs against hydrogen peroxide-induced oxidative damage to corneal endothelial cells: in vitro model.
Presented at: The ASCRS Annual Meeting; March 26, 2011; San Diego, CA.
10. Arshinoff SA. Dispersive-cohesive viscoelastic soft shell technique. J Cataract Refract Surg. 1999;25:167-173.



