INTRODUCTION
By Thomas Samuelson, MD
The glaucoma surgical landscape has evolved substantially in the past few decades. As far back as the 1990s, primary surgery was evaluated in the Moorfields Primary Therapy Trial (MPTT)1 and the Collaborative Initial Glaucoma Treatment Study (CIGTS),2 the former of which demonstrated better visual field outcomes in the laser and surgery groups compared to the medication group. In more modern times, MIGS procedures have begun to replace traditional glaucoma procedures for surgeons around the world, with consistent reductions in rates of trabeculectomy with commensurate increases in rates of MIGS procedures globally.
The OMNI Surgical System (Sight Sciences) is an important evolution in MIGS. It combines two distinct implant-free procedures—trabeculotomy and canaloplasty—in one device and is arguably the most versatile MIGS tool we have based on its multifaceted approach and the degree of angle surgically targeted. OMNI attacks the outflow system in three different ways; it provides 360° direct access to Schlemm's canal and the distal collector channels (Figure 1). It can be used in any stage of glaucoma, and it can be combined with cataract surgery or performed as a standalone procedure.
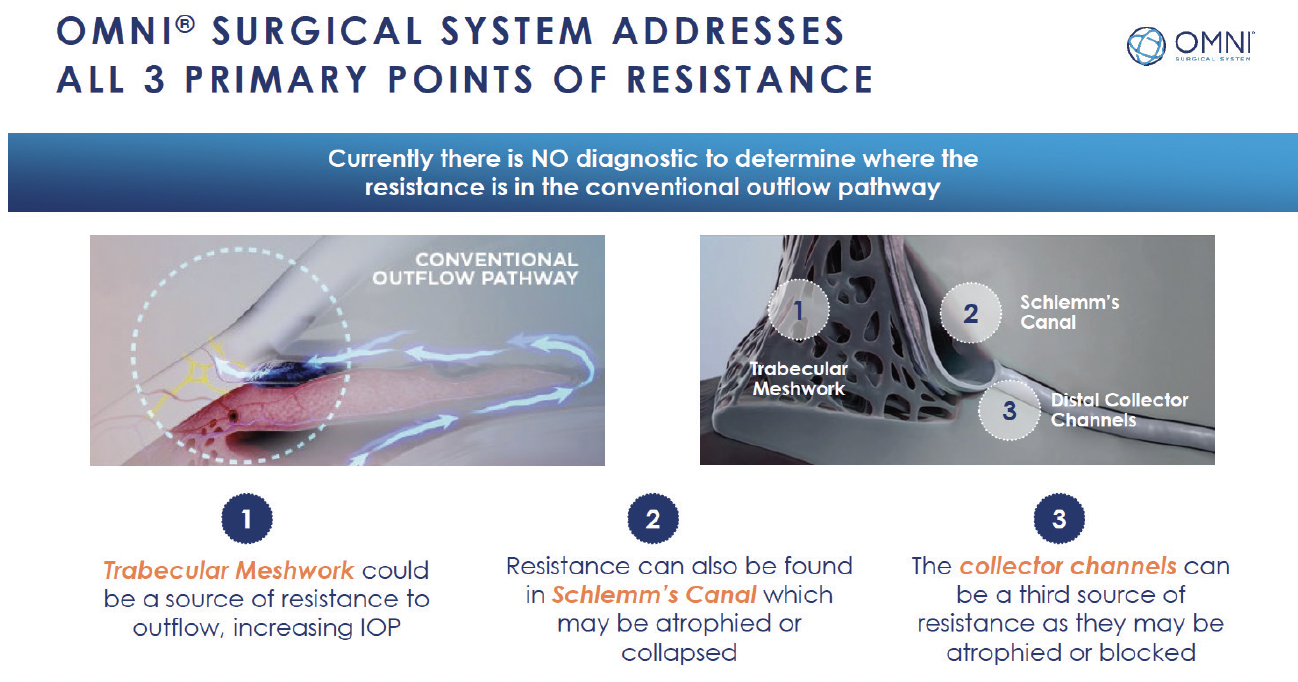
Figure 1. OMNI attacks the outflow system in three different ways.
Potential benefits of early surgical intervention include the reduction and possible elimination of nonadherence to topical medical therapy with better disease control, avoidance of medication-related side effects, and freedom from the hassles of daily self-dosing. The OMNI Surgical System represents a safe and effective ab-interno surgical option for patients with mild to moderate primary open-angle glaucoma (POAG) whose therapeutic goals would otherwise not justify the risk of traditional filtering procedures.
The cases presented here highlight the power of OMNI as a standalone intervention. The procedure is so elegant, and all of us on the panel have had some amazing home runs.
Case No. 1: Expanding the Role of Standalone MIGS
By Shamik Bafna, MD; with commentary from James Katz, MD; Thomas Samuelson, MD; and Zarmeena Vendal, MD
A 75-year-old pseudophakic woman presented with a chief complaint of redness and irritation in both eyes. BCVA was 20/20 OU, she had very mild glaucoma (IOP, 16 mm Hg OD and 17 mm Hg OS), and the cup-to-disc ratio was 0.6 OD and 0.5 OS. She had full visual fields. The patient was on Alphagan (brimonidine tartrate ophthalmic solution 0.1%, AbbVie/Allergan) twice a day. The tear film breakup time was low, but ocular surface issues are not uncommon for patients in their 60s and 70s.
I chose to perform an OMNI procedure on both eyes. Postoperatively, the patient was extremely happy. BCVA was 20/20, the conjunctiva was white and quiet, and IOP was about 18 mm Hg with no drops.
Key Takeaways
In the past, my instinct would have been to change the patient’s medication. Now, I look at a case such as this as an opportunity to perform a MIGS procedure that will potentially reduce or eliminate the medication burden, relieving their symptoms. This, for me, is a big shift in mindset.
Discussion
Dr. Samuelson: I've found the canal-tracking catheter of the OMNI device helps me find and stay within Schlemm’s canal.
Dr. Katz: I also want to point out that angling the catheter up by about 20º or so is helpful to get it on the right pathway.
Case No. 2: Benefits of OMNI in the Pseudophakic Patient
By James Katz, MD; with commentary from Thomas Samuelson, MD, and Zarmeena Vendal, MD
A 78-year-old pseudophakic woman presented for the first time to my clinic with complaints of dry eye. On examination, IOP was 21 mm Hg OD and 24 mm Hg OS. There was some asymmetry on pachymetry (554 µm OD vs 585 µm OS), and the cup-to-disc ratio was 0.3 OD and 0.45 OS. I asked the patient to return in 2 weeks to repeat her pressures, but she never returned. Two years later, the patient returned with a lot more cupping in her left eye versus right (0.35 and 0.65, respectively) and an IOP of 19 mm Hg OD and 31 mm Hg OS.
I started the patient on Lumigan (bimatoprost ophthalmic solution 0.01%, AbbVie/Allergan) in the left eye. The pressure went down initially but crept up again, so I added a second and then a third drop. Selective laser trabeculoplasty (SLT) was performed, but the patient’s IOP returned to the low 20s mm Hg with a cup-to-disc ratio of 0.75 and slight worsening of the visual field.
I decided to perform a standalone 180º canaloplasty and 90º trabeculotomy with the OMNI Surgical System. Postoperatively, the patient has maintained IOPs of 15 to 17 mm Hg for the past 12 months and was able to reduce the medication burden from three drops to two.
Key Takeaways
I am a cornea surgeon and don't do advanced glaucoma procedures, but the OMNI is so intuitive that I don’t have to refer patients to the glaucoma specialist in my practice. I have learned that the key to the procedure is to ensure the microcatheter enters the canal in the right spot. To achieve this, I keep the cannula tip at a 15º to 30º angle. The beveled edge of the cannula is sharp enough to capture the trabecular meshwork. The device stretches out Schlemm’s canal, and as it is retracted, it makes way for the OVD. The deroofing of trabecular meshwork following canaloplasty creates the trabeculotomy.
Discussion
Dr. Samuelson: Another pointer is that it’s important to avoid the limbal vessels whenever you make the small perilimbal corneal incision. Even a small amount of bleeding from a perilimbal vessel will get underneath the gonioprism. I create the incision more anteriorly than I would the clear corneal incision for cataract surgery.
Dr. Vendal: Dr. Katz, do you feel that OMNI helps you to push the envelope and work on cases you otherwise wouldn't have previously?
Dr. Katz: Absolutely. If I didn’t have experience with OMNI, I would have referred the patient to my busy glaucoma specialist after the failed SLT.
Case No. 3: Versatility of OMNI
By Zarmeena Vendal, MD; with commentary from Shamik Bafna, MD; James Katz, MD; and Thomas Samuelson, MD
Case Presentation
A 70-year-old monocular patient with a visual acuity of 20/400 OD due to an eye injury and 20/25-2 OS presented with worsening night vision, especially when driving. He expressed experiencing a lot of glare and terrible dry eye because of his glaucoma drops. His IOP was 20 mm Hg OU, and he had about 0.80 D of astigmatism.
When filtration surgery was the only choice, I would have hesitated to intervene surgically. But with the OMNI, I felt comfortable knowing that the procedure would increase his chance to keep the vision he still had. I therefore decided to combine a 180º canaloplasty and a 180º trabeculotomy with the OMNI. Both procedures are gentle, which was crucial in this case. Toric IOL implantation to correct the patient’s astigmatism was previously performed, demonstrating the ease with which OMNI can be combined with a premium service.
On day 1 postoperative, the patient’s visual acuity was 20/20 and remained at 20/20 at the 1-month follow-up. At that time, his pressure was 8 mm Hg. At 6 weeks postoperative, we withdrew one of his medications and his dry eye improved.
Key Takeaways
Many of us in private practice continually look for ways to diversify our toolbox and increase referrals. The OMNI is an elegant and effective procedure. It can be performed during cataract surgery with either a monofocal or premium IOL, and it can be performed as a standalone procedure. The comprehensive ophthalmologists and optometrists in my community feel comfortable sending their patients to me knowing that the procedure I select will reduce patients’ risk of requiring filtration surgery. It is a very compelling reason to think about taking the leap to perform OMNI as a standalone procedure.
Discussion
Dr. Samuelson: I don't use irrigation and aspiration with a standalone OMNI case, but that is my personal preference. That said, there is good reason to do it; you get to clear any blood from the eye and remove the OVD. For a standalone procedure, however, I irrigate the OVD because there is a benefit to leaving a little bit in the eye to tamponade and prevent hyphema.
Dr. Bafna: Is there a particular OVD that you like to use in standalone cases?
Dr. Samuelson: I like to fill the anterior chamber with a cohesive and then put a bolus of a dispersive within that.
Dr. Katz: I've been doing OMNI for combined cases for so many years. More recently, I began performing it as a standalone procedure and evolved my technique. Now, I leave some OVD inside the eye at the end of the case to prevent hyphema. The procedure itself is relatively straightforward once you learn it. The tips and pearls shared here are crucial to mastering the technique.
Case No. 4: Going Back to the Angle
By Deborah Gess Ristvedt, DO; with commentary from Shamik Bafna, MD; James Katz, MD; Thomas Samuelson, MD; and Zarmeena Vendal, MD
Case Presentation
An 85-year-old man with moderate to severe primary open-angle glaucoma (POAG) in both eyes and a history of multiple glaucoma interventions, including trabeculectomy, continued to experience increasing IOP and an intolerance to different medications over time. He had a mean maximum IOP of 28 mm Hg, thin corneal pachymetry with cup-to-disc ratios of 0.75 OD and 0.8 OS, and was pseudophakic.
At the time of examination, his pressures were in the 20s mm Hg on two medications. He had great central vision, which I wanted him to be able to maintain for his lifetime. I performed a 360º viscocanaloplasty with 360º trabeculotomy. Postoperatively, the patient maintained excellent visual acuity (20/25 OD and 20/20 OS) and a pressure of 10 mm Hg OU on one combination medication in the right eye.
Key Takeaway and Pointers
The key takeaway from this case is that, even in patients who have a history of trabeculectomy, you can go back to the angle.
A few surgical pointers include the following:
- Gonioscopy is key.
- With the trabecular meshwork visualized, approach the angle with the cannula tip, and pierce the meshwork at an angle of 15º to 30º.
- Hold the cannula tip securely against the angle to guide the microcatheter into the canal and to maintain intracameral stability.
- Create a smaller goniotomy; this will avoid excess bleeding and aid in visualization.
- Make sure that you orient your body as you're rotating the bevel to maintain good ergonomics at all times.
- Leave about 20% of the OVD inside the eye to reduce the risk for hyphema.
Discussion
Zarmeena Vendal, MD: Dr. Ristvedt, do you make a second incision to do the second pass with OMNI or do you go through the same incision?
Dr. Ristvedt: I go through the same incision, and I move my microscope and my body to maintain good ergonomics.
Thomas Samuelson, MD: I tend to use the same incision as well. When you first start with OMNI, memorizing the sequence of steps is crucial. If you’re performing a 360º viscocanaloplasty, first go 180º in one direction; perform viscodilation, but don’t incise the inner wall. Then, go forward 180º in the opposite direction, and pull the OMNI back to viscodilate. Then incise the inner wall of TM in both hemispheres.
The tips you shared are great, Dr. Ristvedt. I have one more pearl: I use a myotic—especially for phakic eyes—to keep any bleeding in the anterior chamber as much as possible.
Shamik Bafna, MD: I also use a topical miotic, as well as intracameral lidocaine for standalone procedures.
Dr. Ristvedt: Would others have done a 360º trabeculotomy or a 180º?
James Katz, MD: You got a great result, so I think you did the right thing. I might have done 180º and stripped 90º to 180º. But the patient’s pressure came down, so I think it worked out well for you.
Dr. Bafna: For canaloplasties to have the greatest effect, I find that 360º is best, but for a trabeculotomy 180º can be all that is needed.
Dr. Vendal: It also depends on the patient’s IOP. Are you treating mild, moderate, or severe disease? OMNI is one of the few devices that can be used in mild, moderate, and severe POAG.
Dr. Samuelson: I tend to do quite a bit of 360º trabeculotomy procedures for severe glaucoma because I don't want to think later, 'Gee, should I have done 360º?' I have a different approach for those with mild to moderate glaucoma.
Case No. 5: Preserving Physiological Outflow
By Thomas Samuelson, MD
A 42-year-old man with a history of extreme IOP was referred to my practice for a second opinion. His mean maximum IOP was in the 40s mm Hg OU. He had moderate myopia, a history of SLT, a slightly thin cornea, and a wide open angle. His glaucoma was clearly progressively worsening.
My goal in this case was to preserve physiological outflow. In the past, I would have done a trabeculectomy, but now the OMNI is the best choice for patients such as this one. I performed a 360º canaloplasty followed by trabeculotomy in each eye. Immediately after each surgery, the patient’s pressures dropped significantly. I have followed the patient for more than 2 years, and his recent measurements are as low as 7 to 8 mm Hg OS (Figure 2). It is not typical to see pressures in the low single digits, but it is more common in eyes with thinner corneas. There was some hyphema that didn't clear after about 2 weeks in the first eye, so I irrigated the eye. Since then, he's done great. IOP at his most recent follow-up was 7 mm Hg OU on two drops in his left eye and one drop in his right eye. I will consider withdrawing more at his next follow-up.
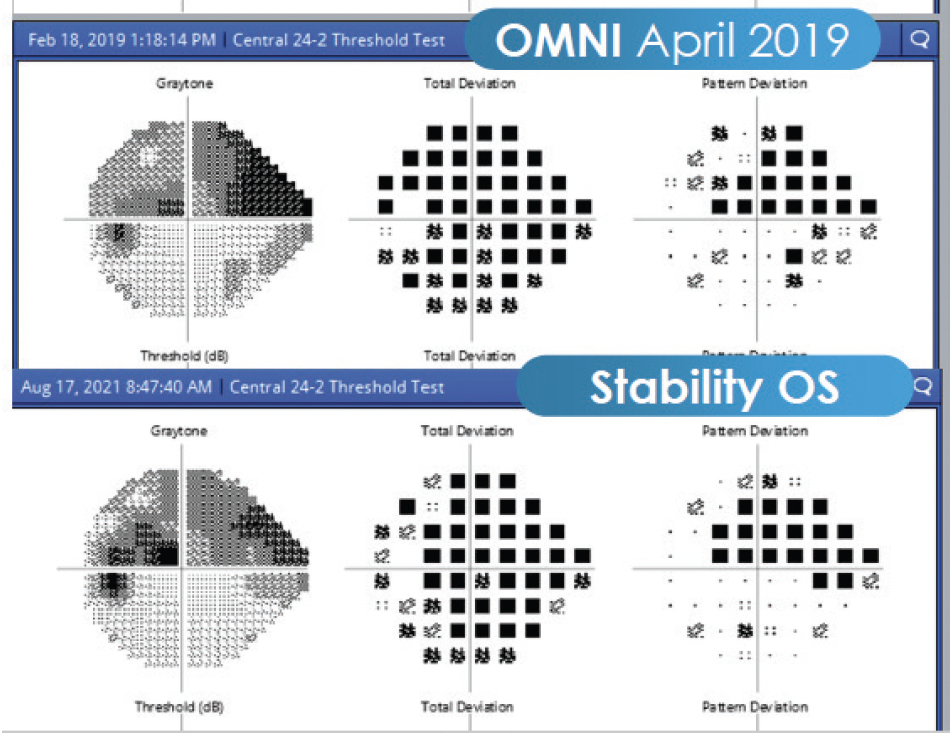
Figure 2. The patient's recent measurements are as low as 7 to 8 mm Hg OS.
Key Takeaways
Keeping the catheter in Schlemm’s canal for the duration of the procedure saves me from having to rethread it again. I also like to perform a walk the dog technique in which the catheter is retracted as you go. It can, however, cause some bleeding and hyphema, which can be exacerbated if the pressure is allowed to be too low. Accordingly, I like to maintain pressure throughout the procedure to prevent excessive reflux.
1. Migdal C, Gregory W, Hitchings R. Long-term functional outcome after early surgery compared with laser and medicine in open-angle glaucoma. Ophthalmology. 1994;101(10):1651-1656.
2. Feiner L, Piltz-Seymour JR, Collaborative Initial Glaucoma Treatment Study. Collaborative Initial Glaucoma Treatment Study: a summary of results to date. Curr Opin Ophthalmol. 2003;14(20:106-111.





