
The pathogenesis of dry eye disease (DED) involves chronic inflammation, triggered by both internal and external factors, which disrupts tear film homeostasis and increases osmolarity.1 The resulting alterations in tear composition create an inflammatory cascade that damages the ocular surface epithelium and triggers neural responses. The last two decades have seen incredible advances in the understanding of the tear film and ocular surface disease (OSD), particularly since the Tear Film and Ocular Surface Society (TFOS) published the first report of the International Dry Eye Workshop (DEWS) in 2007.2
However, there remain significant gaps in patient education and therapeutic tools. Both patients and healthcare providers frequently lack comprehensive understanding of DED’s varied manifestations and chronic nature, which means there will never be a ‘cure’. Challenges with current therapies include lengthy onset times for symptom relief, side effects that are difficult to tolerate, and insurance restrictions that limit access to optimal therapeutic options.
Four eye care practitioners who specialize in the treatment of the ocular surface convened to discuss recently approved and upcoming therapies, why they are excited about them, and how they might use them with their patients.
CURRENT CHALLENGES
Preeya K. Gupta, MD: What are our biggest challenges in treating patients with DED and meibomian gland dysfunction (MGD)?
Marc Bloomenstein, OD: DED presents with myriad different symptoms, it can masquerade as many different conditions, and symptoms often don’t correlate with the signs associated with what we consider classic dry eye. For example, fluctuating vision, difficulty seeing at night, or even the perception that patients always ‘feel’ their eyes. And so, I sometimes find it challenging to get patients to believe me when I diagnose DED, and then to get them to trust me enough to be compliant with a course of treatment that is probably not short or easy.
Another significant challenge is the lack of good diagnostic tools that also function as educational tools. Point-of-care testing to evaluate immunoglobulin E (to differentiate allergy), lactoferrin, or osmolarity in tear fluid would be amazing, and allow us to better tailor both the patient discussion and the individual treatment.
Christopher E. Starr, MD: There are a lot of frustrations and challenges surrounding diagnosing and treating dry eye disease. One big challenge is understanding the role of the corneal nerves and how this can create a discordance between signs and symptoms. The corneal epithelium has the highest density of free nerve endings in the body3 and they are responsible for blinking, tear production, and wound healing mechanisms.4 Yet, we don’t have great everyday tools to measure corneal sensation routinely. As a result, we often try various DED/OSD treatments, and only after those have failed do we start to think about nerve problems. Even for experts in dry eye, corneal nerve damage is easy to miss and often contributes to frustration for the patient and the eyecare practitioner that has stigmatized “dry eye” in the past.
I recently acquired a new non-contact esthesiometer (Brill) and checking corneal sensitivity early in the process is a game changer for my practice. We are now checking corneal sensation proactively on every ocular surface patient as part of the technician workup. It takes my technician under a minute to test both eyes and it doesn’t touch the cornea, unlike more cumbersome, invasive, and time-consuming traditional methods (cotton wisps, dental floss, Cochet-Bonnet esthesiometer, etc.). It has six levels of stimulation (Table 1). Patients with a healthy cornea will respond to level 2 or 3. Levels 4 and 5 are neurotrophic, and at level 6, the patient is essentially anesthetic. What is really interesting is patients who respond to a level 1 stimulus have hypersensitive nerves and possibly a neuropathic corneal pain diagnosis. There is a lot of evidence to show that a hypersensitive corneal nerve is also neuropathic.5 Based on the Brill results, I am now picking up abnormalities of the corneal nerves in many patients who may have been previously diagnosed with DED or another subtype of OSD and treated as such.

Cecelia Koetting, OD, FAAO, DIPABO: I’m so glad you brought this up. It has been reported that DED begins with an increase in nerve density and sensitivity (hyperesthesia) in early stages, followed by a reduction in nerve density and hypoesthesia as the disease progresses.5 Neurotrophic and neuropathic eyes are under-diagnosed, and by catching them early on, we can stop progression to later stages. I am in a targeted OSD clinic, so I am frequently seeing patients with advanced DED. I find it necessary to test corneal sensitivity in about 90% of my patients and have been doing that with the Cochet-Bonnet esthesiometer. My Brill esthesiometer should be arriving soon and I am really interested to see how it works, what the potential applications are, and how it fits into my clinic flow versus the Cochet-Bonnet.
Dr. Bloomenstein: Another benefit to such an easy-to-use esthesiometer is that it allows us to manage patients with neurotrophic keratitis. This is a progressive disease, so I’m excited to be able to diagnose them when they are still at or before stage 1, when I can help prevent disease progression.
Dr. Gupta: I think what you have highlighted is that we really need more diagnostics in dry eye that are accessible to all practitioners. We know that DED is a multifactorial, multi-contributory condition, and I think most clinics across the country don’t even employ the diagnostics we do have. We need the tools and technologies to be easier to implement, less expensive, and less time-consuming to use so that more clinicians incorporate them into their clinical practices.
This ties back to what Dr. Bloomenstein said about education. It’s not only patients, but ongoing peer education. There needs to be more understanding among patients and colleagues that OSD, and in particular DED and MGD, really do impact vision quality. In addition to that, we have medications that require us to ask our patients to wait 90 days to see any improvement. Then we ask our patients to jump through the hoops of insurance. We need more therapies that act rapidly so that we can increase patient compliance and make people feel better faster. As clinicians, that is our number one goal.
RECENTLY APPROVED FDA THERAPIES
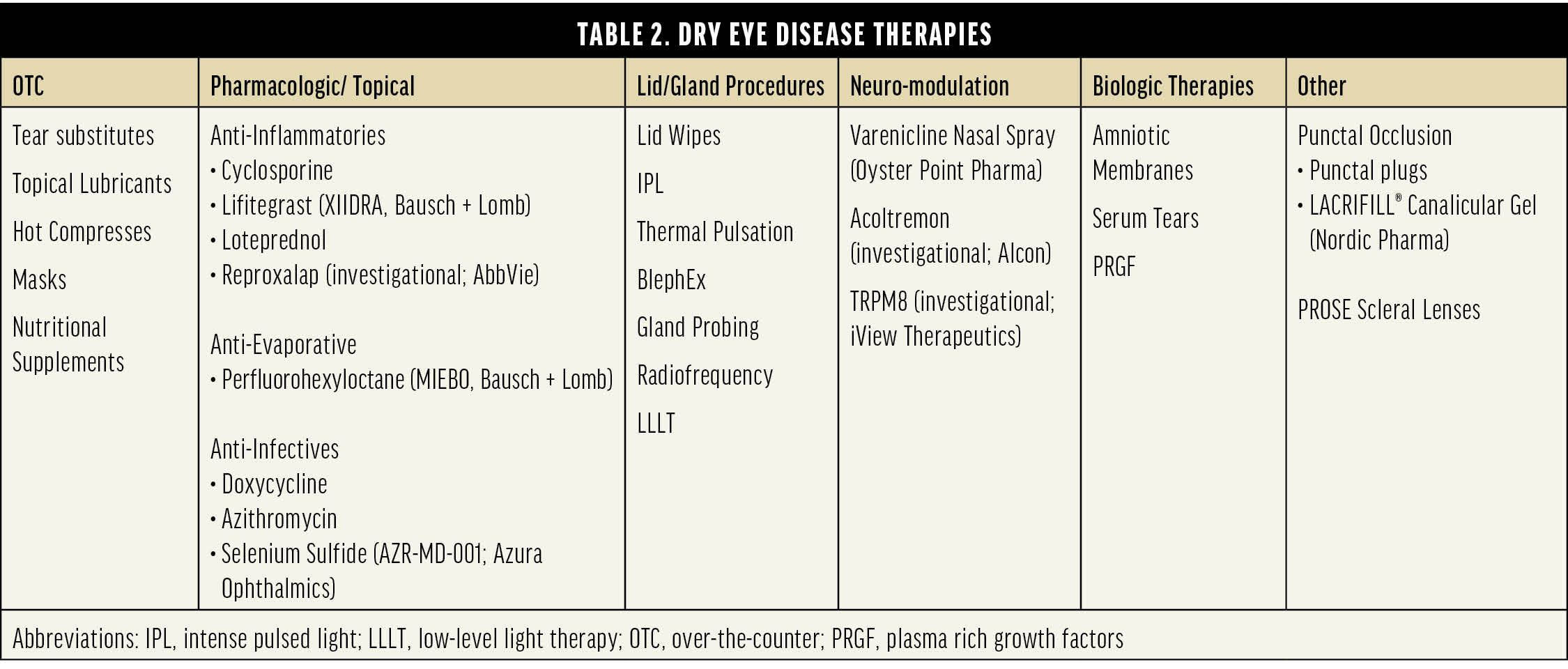
Dr. Gupta: We can divide DED therapies into six broad categories, with all prescription topical drugs in one broad category, sub-divided into anti-inflammatories, anti-evaporatives, and anti-infectives (Table 2). It is worth noting that anti-infectives are used at low doses so that they function as anti-inflammatories. Although not directly related to DED, lotilaner (XDEMVY, Tarsus Pharmaceuticals) for the treatment of Demodex blepharitis should also be thought of in this context—Demodex blepharitis must be treated first, if present, before other therapies will be truly effective.
There are a few therapies that were approved in the last year and some that are in the final stages of approval that are fulfilling some of the needs we have just discussed. Let’s start with LACRIFILL® Canalicular Gel (Nordic Pharma). This is a cross-linked hyaluronic acid hydrogel that completely fills the canalicular system, preventing tears from entering the lacrimal drainage system. It is designed to be left in place for 6 months, when it can be removed by irrigating the canaliculus with saline solution.
Dr. Starr: I was never a big fan of collagen plugs because the patient must return to the clinic so frequently, you’re never quite sure if they are there, and since I usually reserve plugs for patients with very low tear volume, epiphora is typically not a concern. (If it does occur, a non-dissolvable plug is easier to remove than collagen or intracanalicular plugs). LACRIFILL Canalicular Gel, however, is intriguing and so far has been useful in my practice. In a randomized controlled trial comparing LACRIFILL Canalicular Gel to a hydrogel plug, LACRIFILL Canalicular Gel was statistically noninferior and numerically superior in Schirmer test score changes and in proportion of patients achieving improvement in ocular surface disease index (OSDI) scores at 3 months.6 I recently had a patient who needed all four puncta closed. I had been inserting the largest size of silicone plugs available and they kept falling out, frustrating both me and the patient. The LACRIFILL Canalicular Gel hydrogel completely fills the canaliculus, no matter the diameter of the individual patient, so it doesn’t fall out and lasts for 6 months. If it needs to be reversed, I hear the process is fairly easy to flush out. It was a great option for this patient, and I am using it more and more.
Dr. Koetting: I also haven’t loved punctal plugs because they can fall out prematurely, dissolve too quickly, and the permanent plugs often don’t sit flush with the eyelid and punctal area, causing localized discomfort or conjunctival irritation/abrasion. I definitely see a place for LACRIFILL Canalicular Gel with my patients that could benefit from reduced tear outflow.
Another therapy recently approved by the FDA for DED is Vevye (Harrow), which is cyclosporine 0.1%. What has your experience been with this new drop?
Dr. Koetting: My experience with Vevye has been good overall. Clinical trials showed that 56.9% of patients achieved mean improvements of 3 grades or more in corneal staining scores by day 15,7 which is consistent with what I see in my patients. I notice that patients look and feel better about 2 weeks after starting Vevye. It has a great vehicle for a molecule we are already comfortable with, in a higher percentage.
Dr. Bloomenstein: The vehicle in Vevye is perfluorobutylpentane, a water-free semi-fluorinated alkane. Due to its amphiphilic nature, it can effectively dissolve hydrophobic drugs like cyclosporine, dramatically improving the bioavailability of the cyclosporine.8 A big challenge with treating chronic dry eye was getting patients to stay on an immunomodulator long enough to see improvement. My patients taking Vevye seem to stick with it, and I’m seeing the positive clinical results.
Dr. Starr: In a patient who has never been treated for dry eye that has a mix of evaporative MGD plus some aqueous deficiency—the perfect TFOS DEWS II “mixed or hybrid DED”—this is a great first step. I always ask patients how aggressive they want me to be, and in those patients who report they just want one treatment, Vevye offers a potent immunomodulator with a nice lubricating vehicle. It certainly isn’t a substitute for perfluorohexyloctane in evaporative DED, but it is a reasonable first prescription in those with hybrid DED who want minimal intervention.
Dr. Gupta: When I’m making treatment decisions, I am looking for the strongest drug I can give a patient with the least amount of side effects, and Vevye not only has the highest percentage of cyclosporine, but it is also the best tolerated medicine that we have seen in the category. The pooled clinical studies show that 99.8% of patients experienced no or mild instillation site pain.9 This fills a huge need by providing an immunomodulator that is much quicker acting and better tolerated than other options.
PIPELINE THERAPIES
Dr. Gupta: We need more therapies for DED, because different patients respond to different things. Thankfully, there are three novel therapies that have all completed phase 3 studies but are not yet FDA approved. I feel that each of these can potentially shift how we treat DED. Let’s start with Reproxalap (AbbVie; Figure 1). Reproxalap is a reactive aldehyde species (RASP) inhibitor. RASP inhibitors, such as malondialdehyde (MDA), are found in elevated levels in the tears of DED patients and correlate positively with disease severity.10 They are produced by or stimulated by oxidative stress and are what trigger cytokine release and the inflammatory cascade. By intervening earlier in the pathway, we might have a greater impact on DED and a more rapid onset of action. That is novel to this molecule, as well as how it can work in minutes.

Figure 1. Reproxalap showed rapid reduction of ocular discomfort associated with DED in a phase 3 study.11 Image courtesy of Aldeyra.
Dr. Starr: Steroids, immunomodulators, and non-steroidal anti-inflammatory drugs (NSAIDs) each work on a different part of the inflammatory cascade, and Reproxalap works much further upstream than any of these existing classes of medication. It is potentially a much broader and less specific anti-inflammatory medication. It has the efficacy and potency that we expect from a steroid, but looking at trial data, it doesn’t have the side effects that we attribute to steroids, such as intraocular pressure spikes, cataract formation, wound healing inhibition, or higher risk of infection.12
Dr. Bloomenstein: Whenever a new product comes out, I try to look at my patients who are not responding well to their current treatments and consider if the new therapy would be a good fit. I firmly believe that inflammation is the root cause of dry eye in the vast majority of our patients. Steroids work, but the challenge we have with prescribing them is the side effects. I’m excited to try RASP inhibitors. I know there will be a sensation associated with instillation, and I am interested to understand that better as the study does not truly capture patients’ response to this.
Dr. Gupta: Let’s talk about AR-15512 (Acoltremon), a new molecule by Alcon (Figure 2). This is a transient receptor potential melastatin 8 (TRPM8) agonist. Clinical studies have demonstrated that the absence of a TRPM8 gene significantly reduces basal tear secretion and blink rate.13,14 This is a pharmacological agent that neuromodulates, or neurostimulates, the tear pathway and is proving to be a novel pathway and a potent target for increasing basal tear production. Acoltremon works to increase basal tears robustly and also has a very rapid onset of action.

Figure 2. TRPM8 is located on trigeminal sensory nerve terminals in the corneal epithelium. TRPM8 receptors detect drops in temperature associated with tear evaporation on the cornea.15
Dr. Bloomenstein: When we first diagnose a patient with dry eye, we tell them that we need to keep their eyes more lubricated, and we often turn to artificial tears or lubricating drops that are not very similar to the components of basal tears. AR-15512 has the potential to help patients produce their own tears, which makes this incredibly exciting. The clinical trials demonstrated AR-15512 was associated with early and sustained improvements in unanesthetized Schirmer scores.16 The biggest benefit is that patients’ symptoms also improved quickly and continued improvement over time. For me, this will be a first-line treatment and it will be nice to be able to tell patients that they can just use this drop twice per day for sustained basal tear production throughout the day.
Dr. Koetting: The clinical trials showed improved conjunctival staining in addition to improved corneal staining, which is really interesting. Where I see myself using this is both in patients who have not yet started prescription treatment, but also those that are on another treatment and still stop-gapping with artificial tears. I ask all my patients if they are using artificial tears, and if so, how often. I use that as a gauge to know if we are hitting the mark in terms of treatment. If they are still reaching for artificial tears 3 or more times per day, I’m not doing my job correctly and I probably need to adjust their treatment. Currently, this is when I may reach for a nasal spray or varenicline (Tyrvaya, Oyster Point Pharma), a nasal stimulator, or a punctal plug. I would use AR-15512 as an alternative in these patients.
Dr. Gupta: The third new therapy awaiting FDA approval is selenium sulfide ointment, AZR-MD-001 (Azura Ophthalmics), which would be the first topical agent specifically targeting MGD. The clinical trials for AZR-MD-001 were designed to assess twice-weekly application to the lid margin. It features a novel vehicle and has a novel dosing schedule. The selenium sulfide breaks up the keratin bonds within the meibum to allow it to be less viscous. The clinical studies showed improvement in both the number of glands yielding secretions as well as the quality of the oils.17 How would you envision using this therapy in clinical practice?
Dr. Starr: Abnormal keratin production and the aggregation of keratin via disulfide bonds alter meibum quality and viscosity and can ultimately obstruct the flow of meibum out of the glands.18 Selenium sulfide breaks apart the disulfide bonds to soften the blockages and increases the quality and quantity of meibum produced by the meibomian glands,19 so it would fill an unmet need. We don’t currently have a treatment that actually modifies the disease of MGD. We have procedural therapies, such as microblepharoexfoliation (BlephEx LLC), the LipiFlow Thermal Pulsation System (Johnson & Johnson), and TearCare (Sight Sciences) that work really well. However, they are typically performed sporadically and they can be expensive for patients, so they are not available to everyone. Combining AZR-MD-001 with warm compresses, MIEBO (perfluorohexyloctane ophthalmic solution, Bausch + Lomb), and the occasional in-office blepharoexfoliation and thermal pulsation procedure would finally give us the substantial, full-spectrum armamentarium against MGD that we really need.
Dr. Koetting: You hit the nail on the head. I love the in-office treatments, but not all my patients can afford them. We recognize that daily and weekly care are important. That is why we discuss daily eyelid care with warm compresses and lid hygiene, even in patients where we are doing in-office treatments. For that reason, I see the selenium sulfide ointment as a treatment I can do in conjunction with others. I love the fact that it is applied to the eyelids, and it has a triple mechanism of action. It is keratostatic, keratolytic, and lipogenetic, so it helps with multiple aspects of the disease.
Dr. Bloomenstein: Selenium sulfide also affects Demodex mites, so perhaps we’ll discover that using XDEMVY together with this keratolytic is the game changer that we are all looking for.
PATIENT PROFILES
Dr. Gupta: As eye care practitioners who treat DED, we see quite a range of disease severity. For ease, we can divide patients into episodic, chronic, and refractory dry eye (Table 3). Episodic would be a patient reporting a flare in symptoms once a quarter. Chronic could be moderate or severe, but with consistent signs and symptoms. And refractory patients are obviously the most severe. How would you use these new and emerging treatments according to these patient profiles?

Dr. Bloomenstein: In a patient with episodic dry eye, I’m looking to make sure they are keeping their eyes lubricated. I’m excited about some of the newer diagnostic tests since we must determine specifically what pathology is present. The Brill corneal esthesiometer to test corneal sensitivity, and also testing tear osmolarity, really help to dial in these novel treatments. In general, I’m also looking for a therapy that provides the patient quick relief, so maybe a low-dose steroid for a patient having a flare, and/or perfluorohexyloctane if I feel the flare is related to evaporative effects. I’m really focusing on keeping the eyes lubricated, talking about environmental strategies, and reinforcing the fact that they have dry eye disease.
Dr. Koetting: With chronic dry eye patients, whether it is moderate or severe, I am making sure we are creating habits that decrease inflammation. This would include daily lid hygiene, warm compresses, and nutraceuticals. Next, I’m treating any underlying concomitant issues like blepharitis or rosacea. If a patient has Demodex, we really need to get that under control. Finally, I’m keeping them on a long-term anti-inflammatory. That could be cyclosporine or lifitegrast ophthalmic solution 5% (XIIDRA, Bausch + Lomb). This may be where the RASP inhibitor will come in. Going back to what Dr. Starr said, we really must test every dry eye patient for corneal sensitivity and make sure we are monitoring that. If corneal sensitivity is decreasing, you may need to be more aggressive in your treatment, so you don’t end up with refractory DED, or neuropathic or neurotrophic issues.
Dr. Starr: Exactly. Refractory dry eye is probably not dry eye disease in the strictest sense. It’s either multifactorial OSD or some other masquerading issue that is causing the symptoms one might mislabel as DED. The big bucket is corneal nerve abnormalities. Autoimmune diseases, cicatrizing conditions, limbal stem cell deficiency, mechanical eyelid issues, epithelial basement membrane dystrophy, allergic and atopic conditions, etc.—these are all additional etiologies that may need to be addressed in cases of supposed refractory DED.20 This is why I prefer the term OSD, because ‘dry eye’ is generally due to an amalgamation of multiple simultaneous OSDs. Treatment at this point may include things like surgery, amniotic membrane, bandage contact lenses, PROSE lenses, autologous serum drops, tarsorrhaphy, and so on. But I think at this stage, we have to be thinking about corneal nerves and systemic diseases that might be playing a role.
Dr. Gupta: I agree, with refractory disease you must look for masquerading diseases. Diagnostic testing with the esthesiometer will really help with that. The last thing I will add is the importance of assessing the patient’s willingness to be on therapy and their need to be on therapy. It is important to set the expectation that at any stage of DED, you are unlikely to have a ‘cure’. With chronic and refractory dry eye disease, I also set the expectation of polytherapy. Patients frequently come in having tried multiple therapies with the expectation that one thing was going to cure their DED. In reality, these patients need to be on 3, 4, or even 8 to 10 therapies all together to manage their disease. The sooner they accept that, the more successful they are in their treatment. At some point, there is treatment fatigue. This happens more quickly when patients don’t understand their disease, so I find this to be a very important aspect in the spectrum of treatment.
1. Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology. 2017;124(11S):S4–S13.
2. Report of the International Dry Eye WorkShop. Ocul Surf 2007;5[2]:65-206.
3. Marfurt CF, Kingsley RE, Echtenkamp SE. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Invest Ophthalmol Vis Sci. 1989;30(3):461–72.
4. Yang AY, Chow J, Liu J. Corneal innervation and sensation: the eye and beyond. Yale J Biol Med. 2018;91(1):13-21.
5. Al Aqaba MA, Dhillon VK, Mohammed I, et al. Corneal nerves in health and disease. Prog Retin Eye Res. 2019;73:100762
6. Packer M, Lindstrom R, Thompson V, et al. Effectiveness and safety of a novel crosslinked hyaluronate canalicular gel occlusive device for dry eye. J Cataract Refract Surg. 2024;50(10):1051-1057.
7. Akpek EK, Sheppard JD, Hamm A, et al. Efficacy of a new water-free topical cyclosporine 0.1% solution for optimizing the ocular surface in patients with dry eye and cataract. J Cataract Refract Surg. 2024;50(6):644-650.
8. Agarwal P, Khun D, Krösser S, et al. Preclinical studies evaluating the effect of semifluorinated alkanes on ocular surface and tear fluid dynamics. Ocul Surf. 2019;17(2):241-249. .
9. Sheppard JD, Wirta DL, McLaurin E, et al. A Water-free 0.1% cyclosporine a solution for treatment of dry eye disease: results of the randomized Phase 2B/3 ESSENCE Study. Cornea. 2021;40(10):1290-1297.
10. Augustin AJ, Spitznas M, Kaviani N, et al. Oxidative reactions in the tear fluid of patients suffering from dry eyes. Graefes Arch Clin Exp Ophthalmol. 1995;233(11):694-698.
11. Aldeyra Therapeutics Achieves Primary Endpoint in Phase 3 Dry Eye Disease Clinical Trial of Reproxalap. Available at: https://www.businesswire.com/news/home/20240808408806/en/. Accessed February 8, 2025.
12. Clark D, Tauber J, Sheppard J, Brady TC. Early onset and broad activity of Reproxalab in a randomized, double-masked, vehicle-controlled phase 2b trial in dry eye disease. Am J Ophthalmol. 2021;226:22-31.
13. Parra A, Madrid R, Echevarria D, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010;16(12):1396-1399.
14. Quallo T, Vastani N, Horridge E, et al. TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. Nat Commun. 2015;6:7150.
15. Hirata H, Meng ID. Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: Implications for dry eye disease. Invest Ophthalmol Vis Sci. 2010;51(8):3969-3976;
16. Wirta DL, Senchyna M, Lewis AE, et al. A randomized, vehicle-controlled, Phase 2b study of two concentrations of the TRPM8 receptor agonist AT-15512 in the treatment of dry eye disease (COMET-1). Ocul Surf. 2022;26:166-173.
17. Downie LE, Craig JP, Stapleton F, et al. Efficacy and safety of AZR-MD-001 selenium sulfide ophthalmic ointment in adults with meibomian gland dysfunction over six months of treatment: A phase-2, vehicle-controlled, randomized extension trial. Ocul Surf. 2025:35:15-24.
18. Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian glands. Invest Ophthalmol Vis Sci. 2011;52(4):1938-1978
19. Watson SL, Jones LW, Stapleton F, et al; CELESTIAL STUDY Group. Efficacy and safety of AZR-MD-001 selenium sulfide ophthalmic ointment in adults with meibomian gland dysfunction: A vehicle-controlled, randomized clinical trial. Ocul Surf. 2023;29:537-546.
20. Brissette AR, Drinkwater OJ, Bohm KJ, Starr CE. The utility of a normal tear osmolarity test in patients presenting with dry eye disease like symptoms: A prospective analysis. Cont Lens Anterior Eye. 2019 Apr;42(2):185-189. doi: 10.1016/j.clae.2018.09.002. Epub 2018 Sep 17. PMID: 30236650.







