In the United States, topical anti-inflammatory eye drops are the mainstay treatment for dry eye disease (DED).1 Yet, eye care practitioners know that historically, the adherence rate for topical prescription dry eye therapies is quite low. In 2019, White et al concluded that most patients who were prescribed commercially available Restasis® (cyclosporine ophthalmic emulsion) 0.05% (AbbVie/Allergan) and Xiidra® (lifitegrast ophthalmic solution) 5.0% (Bausch + Lomb) discontinued the drops within 12 months (70.8% and 64.4%, respectively).2 In a related study published in 2020, White et al found that a significant proportion of patients using these two medications were dissatisfied with the onset of effect for their treatment, and they often experienced side effects upon instillation (a burning sensation being the most common).3
DED is best managed by continued and consistent treatment. In multiple studies, researchers have found that patients who suffer from DED want a therapy that is comfortable to use, relieves their symptoms quickly, and begins improving their underlying pathology in short order.4-8
CEQUA: A NOVEL FORMULATION OF CYCLOSPORINE
CEQUA (cyclosporine ophthalmic solution) 0.09% (Sun Ophthalmics) was developed to meet patients’ needs for a comfortable, fast-acting, and efficacious topical treatment for DED. CEQUA is a calcineurin inhibitor immunosuppressant that gained FDA approval in 2018 and is indicated to increase tear production in patients with keratoconjunctivitis sicca.9 It is the first and only treatment for DED where cyclosporine A is being delivered with nanomicellar technology (the novel NCELL® Technology), which allows the medication to penetrate the aqueous layer better than non-nanomicellar formulations. In a comparison study, researchers randomized 112 New Zealand white female rabbits to receive either commercially available Restasis or 0.05% CEQUA with NCELL Technology daily for 7 days (note the apples-to-apples comparison of the strength of cyclosporine). The eyes that received cyclosporine with NCELL showed up to three times the amount of drug penetration than the eyes treated with cyclosporine A without NCELL.10

Figure 1. In the phase 3 clinical trial, both the group treated with CEQUA and the group treated with the vehicle demonstrated a mean decrease from baseline of approximately 30% in mSANDE scores. No difference in treatment effect was observed. (Note: Artificial tear use was not permitted during the phase 3 study). Data shown for the ITT population; missing data on day 84 were imputed by baseline values carried forward; P=NS.11,12
IMPROVED CORNEAL STAINING AND VISUAL ACUITY
The clearest demonstration of the efficacy of CEQUA occurred in its phase 2b/3, randomized, multicenter, double-masked, vehicle-controlled study. There, patients who received CEQUA experienced a statistically significant improvement in Schirmer’s scoring (a primary endpoint) of P < 0.01. The researchers evaluated total and central corneal fluorescein staining (CFS) at baseline and on days 14, 28, 42, 56, and 84.11,12 By day 84, CEQUA recipients showed an improvement from baseline of -1.4 (0.09) versus -0.9 (0.09) with the vehicle (P = 0.0002). Also by day 84, the researchers found a significantly high correlation (P = 0.0117) between CEQUA recipients’ reduced central corneal staining and improved Snellen visual acuity.12 Furthermore, Goldberg et al reported that both the CEQUA treatment group (n = 371) and the vehicle treatment group (n = 373) demonstrated a mean decrease from baseline in modified SANDE scores of approximately 30% by day 84 (Figure 1).13
FAST ACTION

Figure 2. Outcomes shown from the phase 2b/3, randomized, multicenter, double-masked, vehicle-controlled, dose-ranging study. The co-primary efficacy endpoints were a mean reduction in the total conjunctival staining score and a mean reduction in the global symptom score at day 84. Conjunctival and corneal staining were assessed at baseline and days 14, 28, 42, 56, and 84/early discontinuation. Conjunctival staining was assessed in 6 conjunctival zones 1–4 minutes after instilling 1 drop of 1% lissamine green. Corneal staining was evaluated in 5 corneal regions 2–2.5 minutes after instilling 1 drop of 0.5% fluorescein.12,14
An important finding in the phase 2b/3 clinical trials was that CEQUA began working within 2 weeks of treatment initiation. In a separate evaluation of patients’ ocular surface endpoints in the clinical trial phase 2b/3 at day 14, those randomized to CEQUA (n = 152) showed least squares mean (SE) change from baseline14 in the following:
- in conjunctival staining, a score of -1.3 (0.1) (vehicle: -1.0 [0.1])14
- in corneal staining, a score of -1.1 (0.17) (vehicle: -0.7 [0.17]) (Figure 2)14
- in tear break-up time, a score of 0.52 (0.15) (vehicle: 0.36 [0.15])14
- in modified SANDE total global symptoms, a score of -4.93 (1.54) (vehicle: -9.1 [1.54])14
- CEQUA 0.09% demonstrated a numerically greater treatment effect compared with vehicle in conjunctival staining, corneal staining, and tear break-up time after 14 days.14
Additionally, by 3 months, 65% of the corneas in the CEQUA group were completely clear, compared to 56.9% in the vehicle group (P = 0.02).13
Safety AND TOLERABILITY
In the CEQUA clinical trials, nearly 95% of those who received CEQUA 0.05% reported experiencing either no or mild instillation site pain after 10 minutes.15 CEQUA is a clear, nonpreserved drop that has a neutral pH. The NCELL Technology of CEQUA is designed to provide comfort and help patients better tolerate cyclosporine.
CEQUA was generally well tolerated in its clinical trials, with most patient-reported adverse events classified as mild or moderate. The most common adverse reactions for CEQUA reported in greater than 5% of patients were pain on instillation of drops (22%) and conjunctival hyperemia (6%). Other adverse reactions reported in 1% to 5% of patients were blepharitis, eye irritation, headache, and urinary tract infection.13
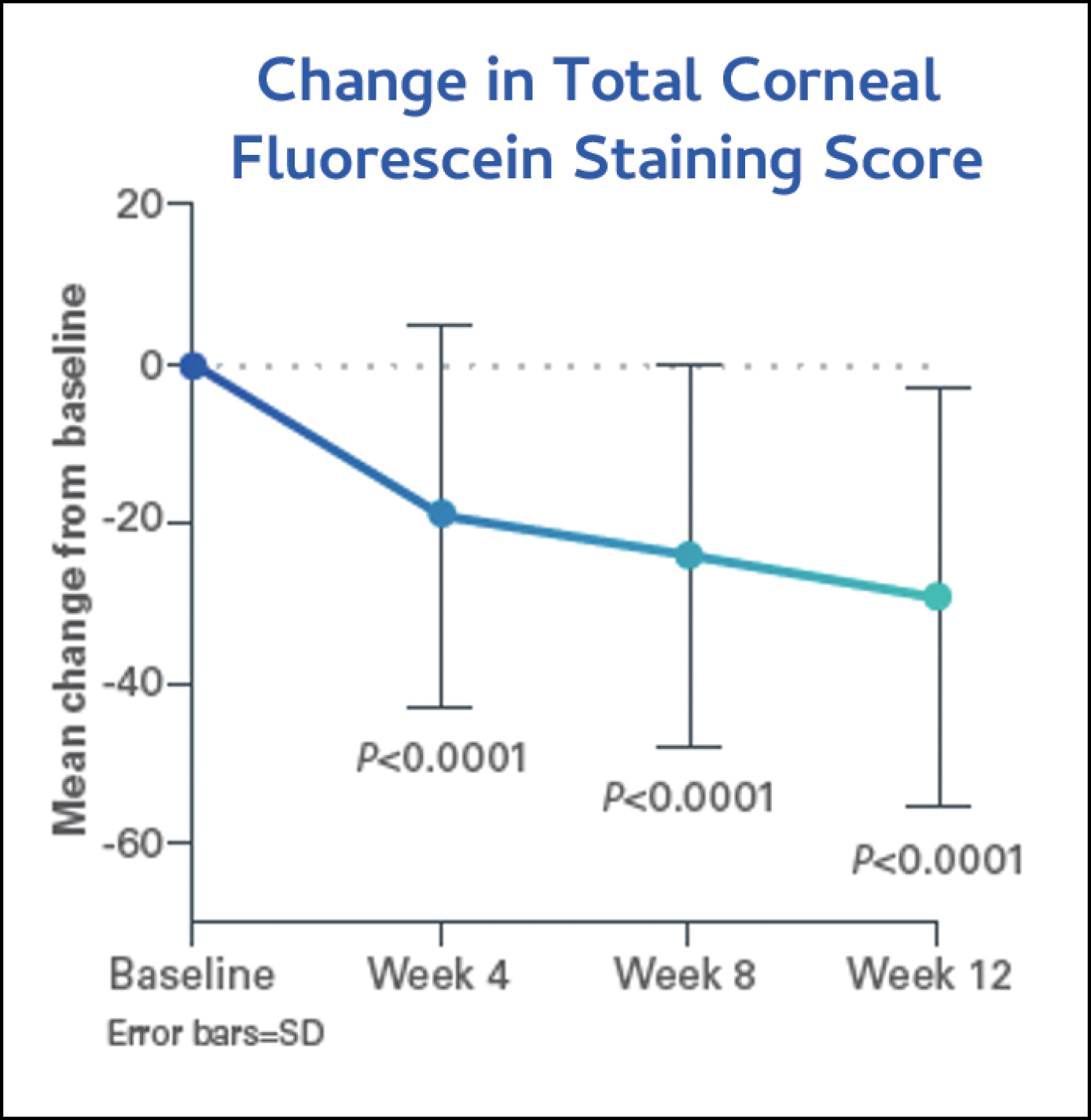
Figure 3. Most patients reported that their eyes felt more comfortable after using CEQUA for 4 weeks than they did using Restasis.16
Notably, CEQUA has no reported taste alterations or contraindications, making it an appropriate option for virtually all adults with DED.12
PHASE 4 DATA: SUSTAINED IMPROVEMENT IN DED SIGNS AND SYMPTOMS IN PATIENTS NOT ADEQUATELY CONTROLLED WITH RESTASIS
At the American Academy of Optometry (AAOpt) annual meeting in October 2023, researchers presented data from the 12-week phase 4 multicenter study of the CEQUA clinical trial.16 Most notably, CEQUA provided sustained amelioration of the signs and symptoms of DED in patients for whom Restasis therapy had failed to control their disease.
The study included 124 adults who had a clinical diagnosis of DED and had been using Restasis for at least 3 months (the average length of Restasis therapy was 38 months), yet who were still symptomatic and/or were exhibiting signs of DED. They received one drop of CEQUA in each eye, twice per day for 12 weeks. Significantly, the study allowed patients to use artificial tears as needed, thereby replicating real-world circumstances in this modified intent-to-treat population.16
The investigators evaluated patients’ CFS and mSANDE scores at baseline and at weeks 4, 8, and 12. Patients showed statistically significant improvements in CFS (Figure 3) and mSANDE scores (Figure 4) in as early as 4 weeks of treatment, and they maintained these improvements through week 12. At baseline, the mean (SD) total CFS score was 5.7 (3.37), which improved significantly (P <0.0001) to 4.0 (3.12) at week 4, 2.9 (2.54) at week 8, and 2.7 (2.36) at week 12. The mean (SD) mSANDE score at baseline was 67.1 (21.05), which also improved significantly (P <0.0001) to 48.4 (23.31) at week 4, 44.2 (24.28) at week 8, and 38.3 (25.99) at week 12. Notably, patients’ artificial tear use dropped from 3x to 1x per day after switching from Restasis to CEQUA for 12 weeks.

Figure 4. Most patients showed statistically significant improvements in total corneal staining as early as week 4, continued to week 12.16
As in its phase 2b/3 clinical trial, CEQUA was generally well tolerated in the phase 4 study. In total, 58 patients (43.3%) reported at least one treatment-emergent adverse event (AE), although most (73.8%) were mild. Instillation site irritation and instillation site pain were the most common treatment-related AEs. No new safety signals appeared in the trial.
SUMMARY
The phase 4 study of the CEQUA clinical trial supported and replicated the outcomes of the phase 2b/3 trials in showing that CEQUA offers a treatment for DED that is at once fast-acting, comfortable, and sustained in its ability to improve the signs and symptoms of the disease.11-16 Assessments of this trial are ongoing, but so far, CEQUA has shown consistent performance in safety and efficacy measurements.

CEQUA and NCELL are registered trademarks of Sun Pharmaceutical Industries Limited. All other trademarks are the property of their respective owners.
PM-US-CQA-1468 02/24
1. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628.
2. White DE, Zhao Y, Ogundele A, et al. Real-world treatment patterns of cyclosporine ophthalmic emulsion and lifitegrast ophthalmic solution among patients with dry eye. Clin Ophthalmol. 2019;22;13:2285-2292.
3. White DE, Zhao Y, Jayapalan H, Machiraju P, Periyasamy R, Ogundele A. Treatment satisfaction among patients using anti-inflammatory topical medications for dry eye disease. Clin Ophthalmol. 2020;14:875-883.
4. Asbell P, Messmer E, Chan C, Johnson G, Sloesen G, Cook N. Defining the needs and preferences of patients with dry eye disease. BMJ Open Ophthalmol. 2019;4(1):e000315.
5. Messmer E, Chan C, Asbell P, Johnson G, Sloesen B, Cook N. Comparing the needs and preferences of patients with moderate and severe dry eye symptoms across four countries. BMJ Open Ophthalmol. 2019;4(1):e000360.
6. Cook NS, Cave J, Holtorf A-P. Patient preference studies during early drug development: aligning stakeholders to ensure development plans meet patient needs. Front Med (Lausanne). 2019;6:82.
7. Ozdemir S, Yeo SWJ, Lee JJ, Bhaskar A, Finkelstein E, Tong L. Patient medication preferences for managing dry eye disease: the importance of medication side effects. Patient. 2022;15(6):679–690.
8. Mah F, Milner M, Yiu S, et al. PERSIST: Physician’s Evaluation of Restasis Satisfaction in Second Trial of topical cyclosporine ophthalmic emulsion 0.05% for dry eye: a retrospective review. Clin Ophthalmol. 2012;6:1971-1976.
9. CEQUA [package insert]. Cranbury, NJ: Sun Pharmaceutical Industries, Inc.; 2022.
10. Weiss SL, William GK. Ocular distribution of cyclosporine following topical administration of OTX-101 in New Zealand white rabbits. J Ocul Pharmacol Ther. 2019; 35(7):395–402.
11. Schechter BA, et al. Effect of OTX-101 on conjunctival and corneal staining at day 14: results of the phase 2b/3 clinical trial. Poster presented at the World Cornea Congress VIII; September 28-29, 2022; Chicago, IL.
12. Malhotra R, Devries DK, Luchs J, et al. Effect of OTX-101, a novel nanomicellar formulation of cyclosporine a, on corneal staining in patients with keratoconjunctivitis sicca: a pooled analysis of phase 2b/3 and phase 3 studies. Cornea. 2019;38:1259-1265.
13. Goldberg DF, Malhotra RP, Schechter BA, Justice A, Weiss SL, Sheppard JD. A phase 3, randomized, double-masked study of OTX-101 ophthalmic solution 0.09% in the treatment of dry eye disease. Ophthalmology. 2019;126:1230-1237.
14. Schechter BA, Urbieta M, Bacharach J, et al. Effect of OTX-101 in patients with dry eye disease at day 14 of treatment: ocular surface endpoint results from the phase 2b/3 clinical trial. Clin Ophthalmol. 2022:16:4145-4151.
15. Tauber J, Schechter BA, Bacharach J, et al. A Phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease. Clin Ophthalmol. 2018;12:1921-1929.
16. Johnston, J. Effect of OTX-101 0.09% on corneal staining and SANDE scores in patients with dry eye disease uncontrolled on cyclosporine ophthalmic emulsion 0.05%. Abstract presented at American Academy of Optometry 2023; October 12, 2023; New Orleans, LA.
INDICATIONS AND USAGE
CEQUA® (cyclosporine ophthalmic solution) 0.09% is a calcineurin inhibitor immunosuppressant indicated to increase tear production in patients with keratoconjunctivitis sicca (dry eye).
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Potential for Eye Injury and Contamination: To avoid the potential for eye injury and contamination, advise patients not to touch the vial tip to the eye or other surfaces.
Use with Contact Lenses: CEQUA should not be administered while wearing contact lenses. If contact lenses are worn, they should be removed prior to administration of the solution. Lenses may be reinserted 15 minutes following administration of CEQUA ophthalmic solution.
ADVERSE REACTIONS
The most common adverse reactions reported in greater than 5% of patients were pain on instillation of drops (22%) and conjunctival hyperemia (6%). Other adverse reactions reported in 1% to 5% of patients were blepharitis, eye irritation, headache, and urinary tract infection.
Please see the Full Prescribing Information.
Important Safety Information
INDICATIONS AND USAGE
CEQUA® (cyclosporine ophthalmic solution) 0.09% is a calcineurin inhibitor immunosuppressant indicated to increase tear production in patients with keratoconjunctivitis sicca (dry eye).
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Potential for Eye Injury and Contamination: To avoid the potential for eye injury and contamination, advise patients not to touch the vial tip to the eye or other surfaces.
Use with Contact Lenses: CEQUA should not be administered while wearing contact lenses. If contact lenses are worn, they should be removed prior to administration of the solution. Lenses may be reinserted 15 minutes following administration of CEQUA ophthalmic solution.
ADVERSE REACTIONS
The most common adverse reactions reported in greater than 5% of patients were pain on instillation of drops (22%) and conjunctival hyperemia (6%). Other adverse reactions reported in 1% to 5% of patients were blepharitis, eye irritation, headache, and urinary tract infection.
Please see the Full Prescribing Information.