Presbyopia remains a universal consequence of aging, an inevitable point when near vision declines and tasks like reading menus, phone screens, or patient charts become noticeably more difficult. Although the condition has traditionally been managed with reading glasses or surgical approaches, recent pharmaceutical advances may cause clinicians to rethink presbyopia treatment.
While earlier pharmacologic efforts in this category launched with excitement, they also came with challenges, particularly around tolerability, headaches, and ocular surface discomfort. According to Marjan Farid, MD, however, low-dose pilocarpine solutions now entering formularies are reshaping the landscape of presbyopia care.
“It’s a space that we sort of started opening a couple of years ago and maybe got off to a rocky start. And I think the horizon is even better now,” Dr. Farid said.
The newest presbyopia drop formulation, preservative-free 0.4% pilocarpine (Qlosi, Orasis), has demonstrated impressive results through both phase 2B1 and phase 3 clinical trials.2 The phase 3 trial met its primary endpoint in showing a significantly greater percentage of responders in the Qlosi group (40.1%) versus vehicle (19.1%; P < .0001) on day 8 at 1 hour post dose 1. In addition, in an exploratory analysis, a greater percentage of patients in the active treatment arm achieved a three-line or better improvement in distance-corrected near visual acuity (Figure 1). Even more impactful, there were relatively few reports of headaches and brow ache, and overall, tolerability was excellent.
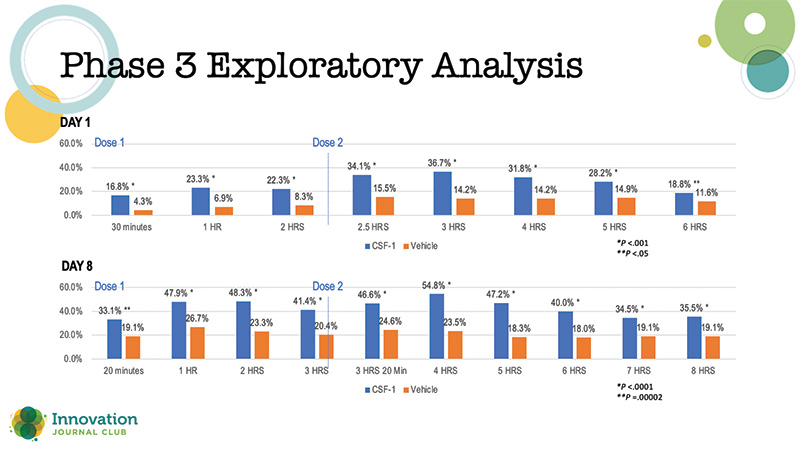
Figure 1. Percentage of responders with ≥ 3-line gain in distance-corrected near visual acuity at day 1 and day 8 in the phase 3 trial of 0.4% pilocarpine (Qlosi, Orasis).
“What really impresses me about the 0.4% pilocarpine formulation, that’s actually now commercially available as Qlosi, is the comfort level. The comfort level is huge, because with the earlier formulation that came out, there was a lot of headaches, there was a lot of irritation to the ocular surface. And I think this proprietary formula being preservative-free is a huge advantage. The comfort level is through the roof,” Dr. Farid said.
Safety remains another central consideration. Although historical use of higher-dose pilocarpine did not show significant increases in retinal detachment risk except among high-risk individuals, responsible prescribing is still key. Access to Qlosi is restricted to eyecare professionals to ensure appropriate screening. For the right patient—Dr. Farid suggested targeting early presbyopes aged 40 to 60—the therapy can offer a nonsurgical bridge before cataract or lens-based options become appropriate.
In addition to selecting the right patients, Dr. Farid said, providers should encourage recipients to give 0.4% pilocarpine time to have its maximum effect. Similar to previous pilocarpine formulations, the effects improve with continued use.
“The study did show that the effects continue to improve over time. There is a little bit of that adjustment,” Dr. Farid said. “I think you tell patients, keep doing it, because it’s going to work even better the longer you’re on it.”
1. Farid M, Rowen SL, Moshirfar M, et al. Combination low-dose pilocarpine/diclofenac sodium and pilocarpine alone for presbyopia: results of a randomized phase 2b clinical trial. Clin Ophthalmol. 2024;18:3425-3439.
2. Holland E, Karpecki P, Fingeret M, et al. Efficacy and safety of CSF-1 (0.4% pilocarpine hydrochloride) in presbyopia: pooled results of the NEAR phase 3 randomized, clinical trials. Clin Ther. 2024;46(2):104-113.

