Introduction
At the annual meeting of the Asia-Pacific Association of Cataract & Refractive Surgeons in June 2023, a distinguished panel of ophthalmologists convened to discuss their current use of the available Alcon IOLs in the Clareon® family, including patient selection and clinical findings.
THE CLAREON® IOL PLATFORM
Dandapani Ramamurthy, MBBS, MD: I have been using the AcrySof® IQ platform (Alcon) for 2 decades. I am extremely comfortable implanting these IOLs. When the Clareon® platform launched, I wondered if it would really make a difference beyond my experience with the AcrySof® material.
The Clareon® IOL is an advanced optic built on the legacy platform of the AcrySof® IQ. The Clareon® incorporates the features of the AcrySof® IQ platform with which surgeons are familiar: asymmetric biconvex optics; STABLEFORCE® haptics for extreme stability;1 a 0º planar configuration between the optic and the haptics; and a 6-mm, full-functioning optic with a square posterior edge that helps significantly reduce posterior capsule opacification (PCO).2,3
Aside from these similarities, there are a few significant differences between the AcrySof® IQ and Clareon® IOL material platforms:
- The Clareon® platform’s manufacturing process involved 59 new validations in its molding, milling, and curing to produce a smoother surface.2,4
- The Clareon® IOL is built on a new, advanced hydrophobic acrylic biomaterial that incorporates HEMA, which results in a slightly higher water content.2,4
The manufacturing advancements further reduced the surface haze characteristics of the IOL for better optical clarity characteristics5 and incorporated a precision edge design. The precision edge design helps guard against PCO development, and the proprietary edge curvature helps minimize the potential for glare and positive dysphotopsia.6,7,8
Clareon® Material and Performance
HEMA uniformly distributes water throughout the polymer matrix of the Clareon® optic, increasing the water content to 1.5% compared to 0.4% in the AcrySof material. This increase in water significantly contributes to the improved optical clarity characteristics of the lens (reducing glistenings and subsurface nanoglistenings).9
In a recent multinational clinical study that involved 19 study sites, Nuijts et al3 evaluated 424 eyes of 215 patients implanted with the Clareon® Monofocal single-piece IOL. They evaluated the primary safety and effectiveness outcomes at 1 year but followed the patients for up to 3 years. The authors concluded that monocular distance BCVA was 20/20 or better in 82% of the patients and 20/25 or better in 93% of the patients implanted with the Clareon® Monofocal IOL (Figure 1). During the 3-year follow-up period, patients maintained visual acuity outcomes as well as MRSE, and 92.9% of the eyes showed no PCO or any clinically non-significant PCO. Only 4.7% of the implanted eyes underwent an Nd:YAG capsulotomy (and several of these occurred at the same site). Moreover, the incidence of reported glistenings in this trial was grade zero in 100% of the eyes, according to the modified Miyata scale*, a clinical finding that reinforces that Clareon® is a glistening-free IOL.
*In this monograph, glistenings are defined per the modified Miyata scale, wherein Grade 0 is equal to <25 MV/mm2
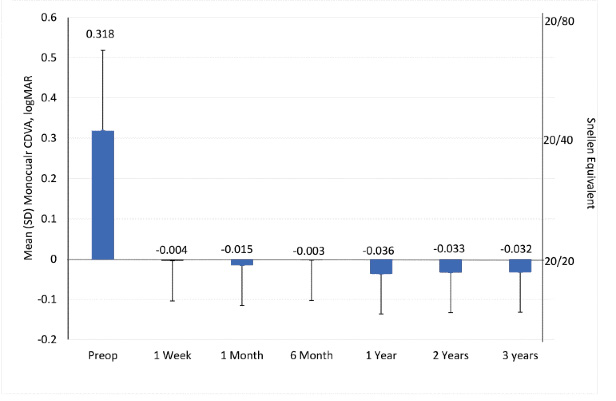
Figure 1. The mean (SD) monocular corrected distance VA (logMAR) at 4 meters (n = 424 eyes) in a clinical study of the Clareon® Monofocal IOL. (Data adapted from Nuijts3 et al. J Cataract Refract Surg. 2023.)
Ben LaHood, MBChB, PGDipOph, PhD, FRANZCO: Importantly, Dr. Ramamurthy, you’ve highlighted that the benefits of the Clareon® Monofocal IOL go beyond an absence of glistenings. I’ve been using the Clareon® Monofocal lens for the past 5 years, and I haven’t seen any glistenings, have you?
Dr. Ramamurthy: I partnered with colleagues in India to conduct a prospective, multicenter trial of the Clareon® Monofocal IOL in which we looked specifically for glistenings in the lenses. According to the modified Miyata scale, amounts of less than 25 MV/mm2 are considered grade zero, and based on this metric, we concluded that the Clareon IOL is glistening-free.10,*
Dr. LaHood: Capsule contraction can move some IOLs from their implantation position to their final location. With its STABLEFORCE® haptics, I believe that the Clareon® lens has good resistance to postoperative movement. Have you found Clareon® Monofocal IOLs to be axially stable?
Dr. Ramamurthy: I agree with you 100%. I have found that the capsule’s dimensions can ultimately determine the final location of the lens. Once the lens adjusts to the capsule, I think it remains there. Clareon® behaves similar to AcrySof with respect to axial stability.11
Dr. LaHood: Have you experienced any refractive surprises? With a stable effective lens position, we wouldn’t expect to see any myopic or hyperopic shifts over time.1,3,12
Dr. Ramamurthy: It stands to reason that the effective lens position of the Clareon® Monofocal lens will not change over time, because of the design of its haptics and the supporting evidence we have seen.3,12 In the past year that my team and I have been using this lens, we have not seen any myopic shifts. Actually, post-market studies of the Clareon® Monofocal IOL with long-term follow-up show consistent refractive stability outcomes. Lehmann et al12 showed that after 1 year of follow-up, subjects achieved a mean MRSE of -0.03 D and 0.04 D at week 1 and month 12, respectively. The cumulative distribution of absolute MRSE in subjects was stable at visits between week 1 and month 12. Absolute MRSE was within 0.50 D in 85.7% (299/349) and 85.1% (291/342) of subjects at week 1 and month 12, respectively.
Likewise, Nuijts et al3 reported that patients’ mean MRSE at week 1 was -0.0021 ± 0.3854 D, and this was sustained through 3 years (0.0973 ± 0.4713 D) (Figure 2). At 3 years, 82.5% (301/365) of eyes presented an absolute MRSE of 0.50 D or less, and 96.7% (353/365) of eyes presented an absolute MRSE of 1.00 D or less.
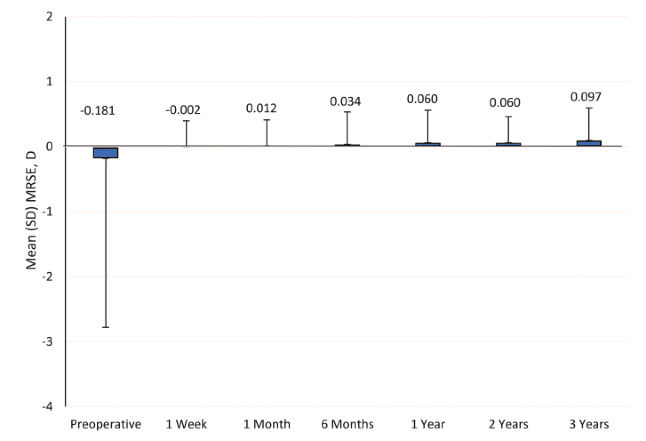
Figure 2. In a multinational clinical study of the Clareon® Monofocal IOL, patients had maintained their mean MRSE values at 3 years postoperatively. (Data adapted from Nuijts et al. J Cataract Refract Surg. 2023.3)
Dr. LaHood: Thank you, Dr. Ramamurthy. My experience with the Clareon® Monofocal IOL so far has been very similar to yours. The results have been very stable.
Visual Outcomes With the Clareon® Monofocal IOL
Dr. LaHood: One interesting clinical observation of which I’ve recently taken greater notice with the Clareon® Monofocal IOL has been that many patients achieve some intermediate vision. I don’t promote the lens as offering intermediate vision, but I have grown to expect that recipients will have more than just clear distance vision.
From my clinical data, I see patients achieve some depth of focus with the Clareon® IOL that is similar to that of some monofocal plus designs, though I have never conducted a comparative trial myself. Previous studies reported the defocus curves of both the AcrySof IQ and Clareon® Monofocal IOLs, showing that with these monofocal lenses we do get some intermediate vision,13-16 and I am definitely seeing it.
Sohee Jeon, MD, PhD: It’s not surprising that these aspheric monofocal IOLs provide some level of depth of focus; in fact, it is likely that we simply didn’t measure intermediate vision before with a monofocal IOL. We can see even in the AcrySof IQ defocus curve that there is some level of intermediate vision possible (approximately 20/32).13,14
David Lubeck, MD: While we cannot promise that the Clareon® or AcrySof IQ® will give intermediate vision, we can say that it gives intermediate vision similar to the monofocal lenses that some manufacturers have tried to position as ‘monofocal plus,’ such as the TECNIS Eyhance IOL (Johnson & Johnson Vision).16
Dr. LaHood: Yes, I agree, Dr. Lubeck. Actually, during the 2023 ASCRS meeting in San Diego, Dr. J. Morgan Micheletti presented the results of a clinical study16 in which colleagues and he compared the Clareon® Monofocal IOL (n = 155) and the TECNIS Eyhance IOL (n = 155). The Clareon® Monofocal IOL showed excellent distance and some intermediate vision, which was clinically no worse than the Eyhance IOL’s intermediate visual acuity. The two IOLs also demonstrated a similar range of vision based on depth of focus curves (Figure 3). In another clinical trial conducted this year, Blehm et al15 also demonstrated a defocus curve for Clareon® similar to the results reported by Dr. Micheletti.
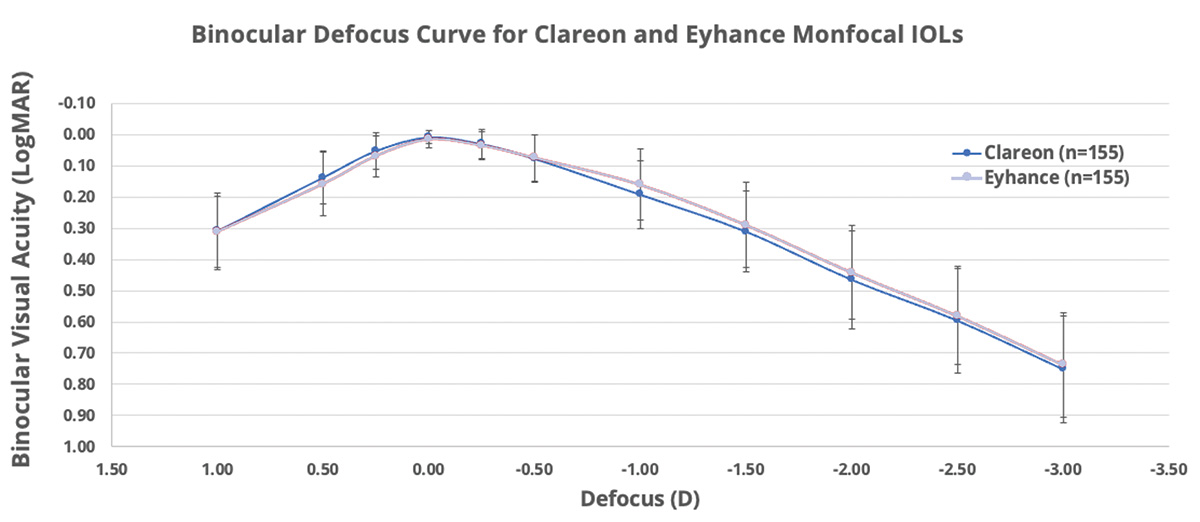
Figure 3. Binocular best distance-corrected defocus curve for Clareon and Eyhance monofocal IOLs. In a clinical study comparing the TECNIS Eyhance monofocal IOL and the Clareon® Monofocal IOL, Micheletti et al found that both lenses provided similar intermediate and distance visual acuities. (Data adapted from Micheletti et al. ASCRS San Diego, 2023.16)
Rotational Stability With The Clareon® Toric IOL
Dr. LaHood: Have any of you had any experience with assessing rotation in the Clareon® Toric IOL? In a study conducted by Walters et al,17 the investigators found less than 5º of rotation in more than 95% of patients implanted with the lens on postoperative day 1 (Figure 4).
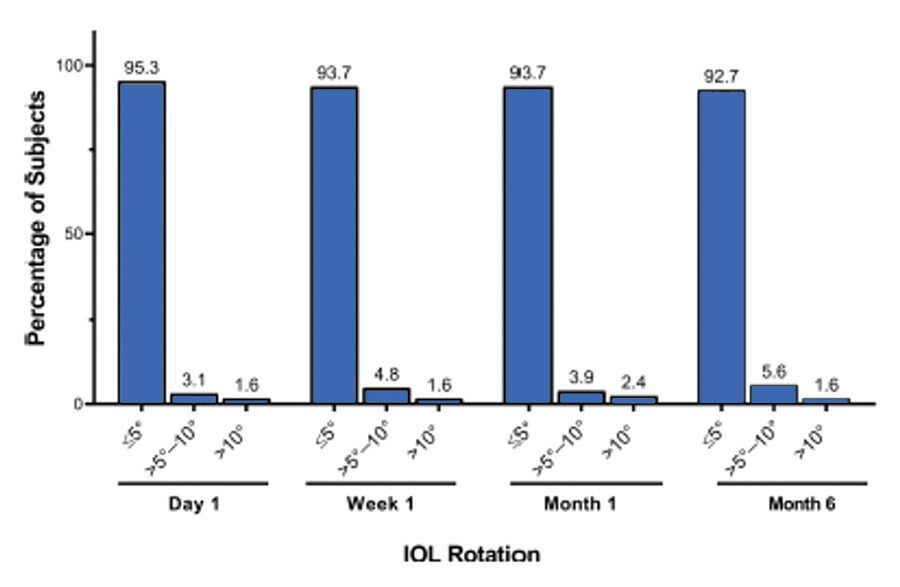
Figure 4. Absolute IOL rotation of ≤5° was observed in 95.3% of subjects on day 1, and 92.7% of subjects at month 6, compared with day 0 (meeting ISO standards). (Data adapted from Walters et al. Clinical Ophthalmology. 2022.17)
Dr. Jeon: I have had no problems with rotation with the Clareon® Toric IOL. I find it to be a very rotationally stable platform, similar to my experience with the AcrySof toric IOL.
Dr. LaHood: I have not had to postoperatively rotate any Clareon® Toric IOL I have implanted. The Clareon® Toric IOL does seem to have a material that demonstrates exceptional bioadhesion, contributing to its rotational stability in the capsular bag. Do you feel the same, Dr. Lubeck?
Dr. Lubeck: For rotational stability, I’ve seen absolutely no difference compared to AcrySof® clinically, and I’ve not had to rotate any IOLs after their initial positioning in the capsular bag. In my experience, the bioadhesive qualities of the Clareon® IOL are as good or better than those of the AcrySof® IOL.18
Dr. Ramamurthy: Thanks to the fibronectin layer that is formed between the lens and the capsular bag, the Clareon® IOL sticks to the capsular bag, which stabilizes its position. That’s why these Clareon® lenses are well established and remain where they are placed. To stabilize an IOL’s position, the lens must stick to the capsule’s back.18
Dr. LaHood: I implanted 80 astigmatic eyes with the Clareon® Toric IOL in a clinical trial and measured their pre- and postoperative astigmatism. Table 1 shows that 94% of these eyes had an overall residual refractive astigmatism of <0.50 D (unpublished data).
For the next 100 eyes I implanted with the Clareon® Toric IOL, on average, I found the IOLs only rotated between the surgery and day 1, and only by an average of 1º. I haven’t seen any rotation occur between day 1 and 1 month after surgery.
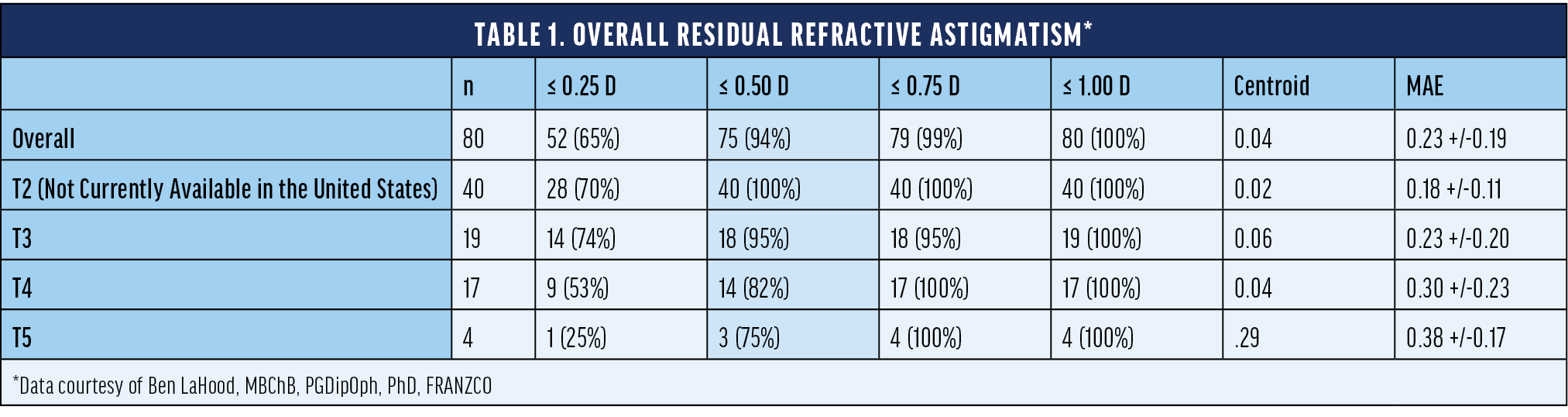
Figure 5 shows the same 100 patients’ intermediate vision with the Clareon® Toric IOL 6 weeks out from surgery. I aimed all of these eyes at a very low minus correction (-0.10 D or -0.20 D). These patients achieved good unaided distance vision—most of them 20/20 or better—and for intermediate visual acuity, more than 50% saw 20/32 or better.
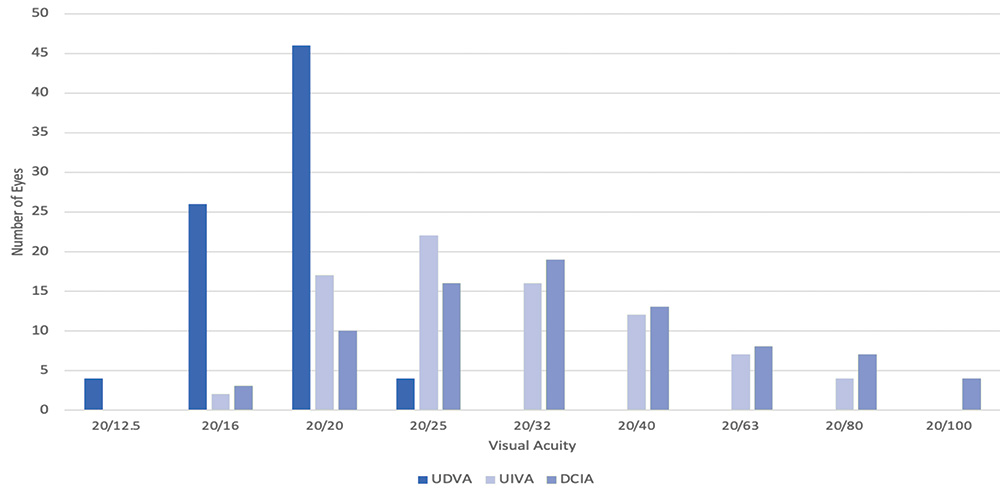
Figure 5. Monocular uncorrected distance visual acuity (UDVA) and uncorrected and distance corrected intermediate visual acuity (UCIVA and DCIVA) for a cohort of 100 eyes implanted with Clareon Toric IOL. Dr. LaHood clinical experience (unpublished data)
THE PRIMARY BENEFITS OF THE PANOPTIX® TRIFOCAL IOL
- More comfortable intermediate vision at 60 cm19,20
- A high degree of spectacle independence21
- A continuous range of vision from distance to near up to 33 cm22
Clareon® PanOptix® Trifocal IOL
Dr. LaHood: I’d like the panel to describe their experiences with the Clareon® PanOptix® IOL.
Dr. Jeon: My team and I have been implanting the Clareon® PanOptix® IOL for the past year. I recently completed a comparison study of the AcrySof® IQ PanOptix® and the Clareon® PanOptix® IOLs in approximately 200 eyes (unpublished data). When we achieve the target refraction, visual acuity is great with both lenses; the only difference we found between the Clareon® PanOptix® and the AcrySof® IQ PanOptix® lenses was slightly better contrast sensitivity with the Clareon® platform.23 The Clareon® PanOptix® IOL is my preferred option among diffractive IOLs. To retina surgeons who are concerned about glistenings associated with the AcrySof material when implanting a diffractive IOL, I recommend the Clareon® PanOptix® lenses. In the past year of implanting this lens, my experience is that the glistening-free* nature of the Clareon® material is an additional benefit to the PanOptix® optical design platform.
In a recent publication, Lee et al23 conducted a retrospective chart review of 135 cataract surgical patients who were 2 months out from implantation with either the AcrySof IQ® PanOptix® IOL or the Clareon® PanOptix® IOL. The visual acuity performance and defocus curve were similar between both lenses. However, contrast sensitivity was significantly better with the Clareon® PanOptix® IOL. According to the subjective photic phenomena survey, patients’ reported degree and incidence of glare was higher for the AcrySof IQ® PanOptix® group.
Such contrast sensitivity differences were not evident between the two material platforms of the existing monofocal IOLs.24 These results suggest that multifocal IOLs may be more optically sensitive than monofocal IOLs, and that the properties of the Clareon® material that give the lens improved clarity can make a functional difference. During the 2-month postoperative follow-up period, no glistenings, surface light scattering, or PCO were observed in any patient. The investigators concluded that the characteristics of the Clareon® IOL platform explained the differences in performance, especially contrast sensitivity, between the two types of lenses.23
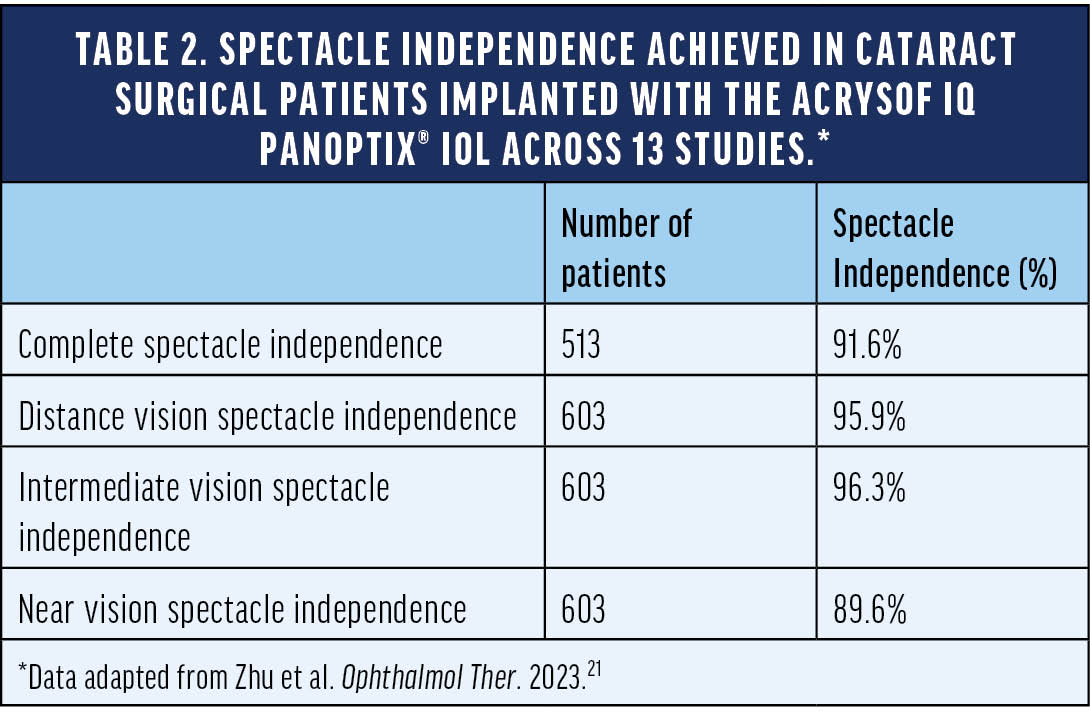
We have a lot of highly myopic patients in East Asia, so sometimes they need an IOL capable of providing a near range of vision up to 33 cm. I think we can achieve spectacle independence in most eyes implanted with the Clareon® PanOptix® IOL if we are on target (within +/-0.25 D) with our refraction. In a systemic literature review and meta-analysis of 13 studies, Zhu et al21 found that patients’ rate of complete spectacle independence after receiving the AcrySof IQ PanOptix® IOL during cataract surgery was 91.6%. Table 2 shows the breakdown of spectacle independence at distance, intermediate, and near.
Dr. LaHood: In my clinical opinion, the PanOptix® IOL gives the full range of vision from near to distance. A study published by Kohnen et al22 supported my clinical experience by demonstrating the continuous range of vision for the AcrySof IQ PanOptix® trifocal IOL in a pooled analysis from 6 prospective clinical trials worldwide (Figure 6). This analysis offers additional evidence of the ability of the PanOptix® lens to provide clear vision from distance to near at 33 cm.
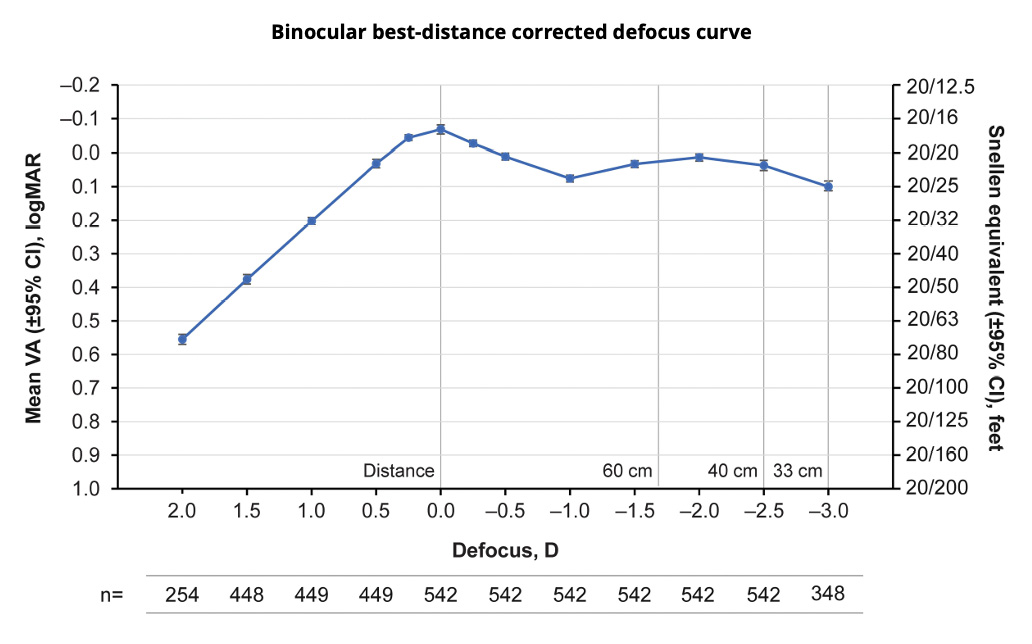
Figure 6. This binocular best distance-corrected defocus curve shows that the AcrySof IQ PanOptix® lens provides a continuous range of vision from distance to near at 33 cm above 20/25 Snellen visual acuity. (Data adapted from Kohnen et al. Clinical Ophthalmology. 2023.22)
Hiroko Bissen-Miyajima, MD: With the introduction of the Clareon® platform on Alcon’s presbyopia-correcting IOLs, I feel comfortable implanting these lenses in my patients. I was very happy with the function of the AcrySof IQ® PanOptix® IOL, but glistenings were a significant concern among Japanese surgeons. Now, all the benefits of the AcrySof IQ PanOptix® and AcrySof IQ Vivity® lenses are built on a glistenings-free Clareon IOL platform, which helps to mitigate those historical concerns.3
The three key characteristics that I would like to highlight about the Clareon® PanOptix® IOL are its stable visual outcomes over time, its continuous range of uncorrected vision from distance to near, and the toric version of the lens.
THE CLAREON Vivity® / ACRYSOF IQ VIVITY® IOL
Dr. LaHood: Dr. Lawless, tell us about your work on the Vivity Registry study,25 which examined real-life outcomes from patients implanted with the AcrySof IQ Vivity® IOL.
STUDY OUTCOMES
- Primary effectiveness endpoint
- Photopic binocular uncorrected VA at distance
- Exploratory effectiveness endpoints
- Photopic binocular distance corrected VA at distance, intermediate (66 cm), and near (40 cm) distances
- Residual refractive error
- Patient satisfaction (CATQUEST- 9SF) and spectacle independence (IOLSAT)
- Safety endpoint
- Visual disturbances (non-prompted interview)
Michael Lawless, MBBS, FRANZCO, FRACS: The Vivity Registry Study used data amassed from practices in several European countries, plus Australia and New Zealand. The study included 810 real-world patients who had cataracts and were implanted bilaterally with the AcrySof IQ Vivity® IOL, toric and non-toric models, without postoperative enhancements. Approximately 30% of them had comorbidities: mostly dry eye; some macular pathology. Around 4% of the patients had undergone previous LASIK surgery.25
To summarize the outcomes, the subjects’ distance UCVA averaged 20/20, intermediate UCVA averaged 20/25, and near UCVA averaged 20/32.25 Nearly 60% of patients reported never or rarely needing to use spectacles for near vision, depending on the lighting conditions. More than 90% showed zero (unsolicited) photic phenomenon: no glare, no starbursts, no halos. Approximately one-quarter of the subjects received a form of mini-monovision—sometimes intended, sometimes not intended—that helped their near vision performance. Despite that, overall, the group experienced quality distance vision without photic phenomenon. These data tell us that the AcrySof IQ Vivity® lens performs as it was intended to.
Dr. LaHood: Dr. Lawless, you have significant experience implanting the AcrySof IQ Vivity® lens. What is your personal experience?
Dr. Lawless: My practice is very much a refractive cataract practice. Most of my patients are between 50 and 70 years old, and around 25% of the eyes from which I remove cataracts have undergone previous refractive surgery. The AcrySof IQ Vivity® IOL has been very successful in my practice.
Dr. LaHood: Do you routinely measure higher-order aberrations? I’m thinking mainly spherical aberration for these eyes. Do you take that into account?
Dr. Lawless: Yes, I measure every cornea for higher-order aberrations. Not the entire eye, because these patients have cataracts. Spherical aberration is one thing, but more importantly, I look at coma and trefoil, which can detract from high-quality vision as much as a lot of spherical aberration. In some cases, coma and trefoil may lead to photic phenomena that are harder to tolerate than spherical aberration, which is usually associated with halos around lights. The AcrySof IQ Vivity® IOL allows me to implant a PCIOL with low levels of dysphotopsia.13,14 I use the Vivity® IOL in about 50% of post-LASIK patients. If I assess the cornea to be too compromised from the original LASIK, I will use a Clareon® Monofocal IOL. The corneal higher-order aberration assessment helps me in this regard.
Prof. Bissen-Miyajima: The Clareon® Vivity® IOL is assisting my clinical practice. I prefer this lens for patients who have concerns about halos and glare, or those with mild ocular comorbidities. Prior to the Clareon® Vivity® IOL, I was implanting monofocal lenses in those patients, but now they can benefit from a presbyopia-mitigating IOL.13
†The safety and efficacy of Vivity™ has not been established in patients with comorbidities. Sound clinical judgment should be used before implanting Vivity™ in these patients.
Dr. LaHood: Do any of you consider implanting the Clareon® Vivity® IOL in patients who have some mild ocular comorbidities, apart from the eyes which have undergone corneal refractive surgery?
Dr. Jeon: I always prefer the AcrySof IQ Vivity® IOL over diffractive IOLs for patients with early epiretinal membrane, because the retinal membrane will eventually get worse with age. My results from a clinical trial26 of the AcrySof IQ Vivity® IOL in eyes with and without low-grade epiretinal membrane demonstrated similar uncorrected and corrected distance visual acuity, as well as dysphotopsia between groups. Patients with mild epiretinal membrane can benefit from the AcrySof IQ Vivity® IOL, because it gives them an extended range of vision with low incidences of visual dysphotopsia.
Dr. Lubeck: The comorbidities† for which I would consider the Clareon® Vivity® IOL include mild ocular surface disease, Fuchs dystrophy without corneal edema, mild epiretinal membrane, and mild-to-moderate glaucoma without significant nerve changes or ganglion cell loss. All of these types of cases are included in my cohort of first 100 Clareon® Vivity® subjects, which includes a mix of healthy eyes, a variety of comorbidities, and post-refractive surgery eyes (unpublished data). All patients are implanted bilaterally, with the dominant eye targeted for the first minus on biometry and the nondominant eye for the second minus.
CONCLUSION
Dr. LaHood: Clareon® is a new glistening-free IOL* material that has improved on the familiar AcrySof platform. The Clareon® Monofocal IOL shows excellent distance vision quality. It has great refractive predictability, with stable outcomes over a long period of time.3 Clinically, some surgeons are now measuring and documenting intermediate vision in patients implanted with the Clareon® Monofocal lens, similar to other monofocal IOLs such as the TECNIS Eyhance.13-16
The PanOptix® Trifocal IOL provides a more comfortable, 60-cm range of intermediate vision, which is particularly good for patients with shorter arm lengths.19,20 It demonstrates very high spectacle independence—at least 9 out of 10 patients do not need glasses,21 and it provides good vision all the way up to near vision at 33 cm.22
The AcrySof IQ Vivity® IOL offers patients excellent distance and intermediate vision, and functional near vision, with very low visual disturbances.13,14,25 The Clareon® Vivity® IOL could be a good option for those who are not eligible for the PanOptix® IOL, even in eyes with some mild comorbidities.†
With the Clareon® PanOptix® and Clareon® Vivity® lenses being built on the Clareon® IOL platform, we now have the same optical clarity benefits as the Clareon® Monofocal lens. My colleagues and I look forward to using all these IOL options.
Important Product Information - Clareon® Family of IOLs
CAUTION: Federal law restricts these devices to sale by or on the order of a physician.
INDICATION: The family of Clareon® intraocular lenses (IOLs) includes the Clareon® Aspheric Hydrophobic Acrylic and Clareon® Aspheric Toric IOLs, the Clareon® PanOptix® Trifocal Hydrophobic IOL, Clareon® PanOptix® Toric, Clareon® Vivity™ Extended Vision Hydrophobic Posterior Chamber IOL and Clareon® Vivity™ Toric IOLs. Each of these IOLs is indicated for visual correction of aphakia in adult patients following cataract surgery. In addition, the Clareon® Toric IOLs are indicated to correct pre-existing corneal astigmatism at the time of cataract surgery. The Clareon® PanOptix® lens mitigates the effects of presbyopia by providing improved intermediate and near visual acuity, while maintaining comparable distance visual acuity with a reduced need for eyeglasses, compared to a monofocal IOL. The Clareon® Vivity™ lens mitigates the effects of presbyopia by providing an extended depth of focus. Compared to an aspheric monofocal IOL, the lens provides improved intermediate and near visual acuity, while maintaining comparable distance visual acuity. All of these IOLs are intended for placement in the capsular bag.
WARNINGS/PRECAUTIONS:
General cautions for all Clareon® IOLs:
Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting any IOL in a patient with any of the conditions described in the Directions for Use that accompany each IOL. Physicians should target emmetropia, and ensure that IOL centration is achieved.
For the Clareon® Aspheric Toric, PanOptix® Toric and Vivity™ Toric IOLs, the lens should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation.
For the Clareon® PanOptix® IOL, some visual effects may be expected due to the superposition of focused and unfocused multiple images. These may include some perceptions of halos or starbursts, as well as other visual symptoms. As with other multifocal IOLs, there is a possibility that visual symptoms may be significant enough that the patient will request explant of the multifocal IOL. A reduction in contrast sensitivity as compared to a monofocal IOL may be experienced by some patients and may be more prevalent in low lighting conditions. Therefore, patients implanted with multifocal IOLs should exercise caution when driving at night or in poor visibility conditions. Patients should be advised that unexpected outcomes could lead to continued spectacle dependence or the need for secondary surgical intervention (e.g., intraocular lens replacement or repositioning). As with other multifocal IOLs, patients may need glasses when reading small print or looking at small objects. Posterior capsule opacification (PCO), may significantly affect the vision of patients with multifocal IOLs sooner in its progression than patients with monofocal IOLs.
For the Clareon® Vivity™ IOL, most patients implanted with the Vivity™ IOL are likely to experience significant loss of contrast sensitivity as compared to a monofocal IOL. Therefore, it is essential that prospective patients be fully informed of this risk before giving their consent for implantation of the Clareon® Vivity™ IOL. In addition, patients should be warned that they will need to exercise caution when engaging in activities that require good vision in dimly lit environments, such as driving at night or in poor visibility conditions, especially in the presence of oncoming traffic. It is possible to experience very bothersome visual disturbances, significant enough that the patient could request explant of the IOL. In the parent AcrySof® IQ Vivity™ IOL clinical study, 1% to 2% of AcrySof® IQ Vivity™ IOL patients reported very bothersome starbursts, halos, blurred vision, or dark area visual disturbances; however, no explants were reported.
Prior to surgery, physicians should provide prospective patients with a copy of the Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with these IOLs.
ATTENTION: Reference the Directions for Use labeling for each IOL for a complete listing of indications, warnings and precautions.
Important Product Information - AcrySof® Family of IOLs
CAUTION: Federal law restricts these devices to sale by or on the order of a physician.
INDICATION: The family of AcrySof® single-piece intraocular lenses (IOLs) includes AcrySof® UV-absorbing IOLs (“AcrySof® UV”), AcrySof® IQ, AcrySof® IQ Toric, AcrySof® IQ ReSTOR®, AcrySof® IQ ReSTOR® Toric, AcrySof® IQ PanOptix®, AcrySof® IQ PanOptix® Toric, AcrySof® IQ Vivity™ and AcrySof® IQ Vivity™ Toric IOLs. Each of these IOLs is indicated for visual correction of aphakia in adult patients following cataract surgery. In addition, the AcrySof Toric IOLs are indicated to correct pre-existing corneal astigmatism at the time of cataract surgery. The AcrySof IQ ReSTOR IOLs are for cataract patients with or without presbyopia, who desire increased spectacle independence with a multifocal vision. The AcrySof® IQ PanOptix® lens mitigates the effects of presbyopia by providing improved intermediate and near visual acuity, while maintaining comparable distance visual acuity with a reduced need for eyeglasses, compared to a monofocal IOL. The AcrySof® IQ Vivity™ lens mitigates the effects of presbyopia by providing an extended depth of focus. Compared to an aspheric monofocal IOL, the lens provides improved intermediate and near visual acuity, while maintaining comparable distance visual acuity. All of these IOLs are intended for placement in the capsular bag.
WARNINGS/PRECAUTIONS:
General cautions for all AcrySof® and AcrySof® UV IOLs:
Careful preoperative evaluation and sound clinical judgment should be used by the surgeon to decide the risk/benefit ratio before implanting any IOL in a patient with any of the conditions described in the Directions for Use that accompany each IOL. Caution should be used prior to lens encapsulation to avoid lens decentration or dislocation. Physicians should target emmetropia, and ensure that IOL centration is achieved.
Additional Cautions associated with AcrySof® IQ ReSTOR® and AcrySof® IQ PanOptix® IOLs: Some patients may experience visual disturbances and/or discomfort due to multifocality, especially under dim light conditions. These may include some perceptions of halos or starbursts, as well as other visual symptoms. Therefore, patients implanted with multifocal IOLs should exercise caution when driving at night or in poor visibility conditions. A reduction in contrast sensitivity may occur in low light conditions. Spectacle independence rates vary with all multifocal IOLs; as such, some patients may need glasses when reading small print or looking at small objects. Patients should be advised that unexpected outcomes could lead to continued spectacle dependence or the need for secondary surgical intervention (e.g., intraocular lens replacement or repositioning). Posterior capsule opacification (PCO), may significantly affect the vision of patients with multifocal IOLs sooner in its progression than patients with monofocal IOLs.
Additional Cautions associated with AcrySof® IQ Vivity™ IOL:
Most patients implanted with the AcrySof® IQ Vivity™ IOL are likely to experience significant loss of
contrast sensitivity as compared to a monofocal IOL. Therefore, it is essential that prospective patients be
fully informed of this risk before giving their consent for implantation of the AcrySof® IQ Vivity™ IOL. In
addition, patients should be warned that they will need to exercise caution when engaging in activities that require good vision in dimly lit environments, such as driving at night or in poor visibility conditions,
especially in the presence of oncoming traffic. It is possible to experience very bothersome visual disturbances, significant enough that the patient could request explant of the IOL. In the AcrySof® IQ Vivity™ IOL clinical study, 1% to 2% of AcrySof® IQ Vivity™ IOL patients reported very bothersome starbursts, halos, blurred vision, or dark area visual disturbances; however, no explants were reported.
Additional Cautions associated with AcrySof® IQ Toric, AcrySof® UV Toric ReSTOR® Toric, AcrySof® IQ PanOptix® Toric, and AcrySof® IQ Vivity™ Toric IOLs: Optical theory suggests that, high astigmatic patients (i.e. > 2.5 D) may experience spatial distortions. Possible toric IOL related factors may include residual cylindrical error or axis misalignments. Toric IOLs should not be implanted if the posterior capsule is ruptured, if the zonules are damaged, or if a primary posterior capsulotomy is planned. Rotation can reduce astigmatic correction; if necessary lens repositioning should occur as early as possible prior to lens encapsulation. Prior to surgery, physicians should provide prospective patients with a copy of the appropriate Patient Information Brochure available from Alcon informing them of possible risks and benefits associated with the these IOLs.
ATTENTION: Refer to the Directions for Use labeling for the specific IOL for a complete list of indications, warnings and precautions.
1. Lane S, Collins S, Das KK, et al. Evaluation of intraocular lens mechanical stability. J Cataract Refract Surg. 2019;45:501-506.
2. Clareon® DFU. Alcon Data on File 2018.
3. Nuijts RMMA, Bhatt U, Nanvaty MA, et al. Three-year multinational clinical study on an aspheric hydrophobic acrylic intraocular lens. J Cataract Refract Surg. 2023;49(7):672-678.
4. Alcon Data on file 2016
5. Alcon Data on File 2014
6. Alcon Data on File 2017
7. Alcon Data on File 2017
8. Das KK, Werner L, Collins S, Hong X. In vitro and schematic model eye assessment of glare or positive dysphotopsia-type photic phenomena: Comparison of a new material IOL to other monofocal IOLs. J Cataract Refract Surg. 2019;45(2):219-227.
9. Werner L, Thatthamla I, Ong M, et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. J Cataract Refract Surg. 2019;45:1490–1497.
10. Titiyal JS, Basak SK, Shetty N, et al. Twelve-months follow-up postmarket study of a hydrophobic intraocular lens using a preloaded automated injector in an Indian population. Clin Ophthalmol. 2022;16:4215–4225.
11. Ullrich M, Ruiss M, Hienert J, et al. Anterior chamber depth variability between 2 hydrophobic acrylic 1-piece intraocular lenses: randomized trial. J Cataract Refract Surg. 2021;47:1460–1465.
12. Lehmann R, Maxwell A, Lubeck DM, Fong R, Walters TR, Fakadej A. Effectiveness and safety of the Clareon monofocal intraocular lens: outcomes from a 12-month single-arm clinical study in a large sample. Clin Ophthalmol. 2021;15;1647–1657.
13. Bala C, Poyales F, Guarro M, et al. Multicountry clinical outcomes of a new nondiffractive presbyopia-correcting IOL. J Cataract Refract Surg. 2022;48:136-143.
14. McCabe C, Berdahl J, Reiser H, et al. Clinical outcomes in a U.S. registration study of a new EDOF intraocular lens with a nondiffractive design. J Cataract Refract Surg. 2022;48:1297-1304.
15. Blehm C, Hall B. Evaluation of visual outcomes and 3-month refractive stability of a new hydrophobic acrylic intraocular lens. Clin Ophthalmol. 2023;17:1859–1864.
16. Micheletti JM, Duncan N, Hall B. Head-to-head comparison of intermediate vision of two monofocal intraocular lenses. Presented at the American Society of Cataract and Refractive Surgery (ASCRS) Annual Meeting; May 5-8, 2023; San Diego, CA.
17. Walters TR, Lehmann R, Moyes A, et al. Rotational stability of the Clareon® monofocal aspheric hydrophobic acrylic intraocular lens 6 months after implantation. Clin Ophthalmology. 2022;16: 401–409.
18. Alcon Data on File 2017
19. Modi S, Lehmann R, Maxwell A, et al. Visual and patient-reported outcomes of a diffractive trifocal intraocular lens compared with those of a monofocal intraocular lens. Ophthalmology. 2021;128(2):197-207.
20. Lwowski C, Pawlowicz K, Petermann K, et al. Visual and patient-reported factors leading to satisfaction after implantation of diffractive extended depth-of-focus and trifocal intraocular lenses. J Cataract Refract Surg. 2022;48(4):421–428.
21. Zhu D, Ren S, Mills K, Hull J, Dhariwal M. Rate of complete spectacle independence with a trifocal intraocular lens: a systematic literature review and meta-analysis. Ophthalmol Ther. 2023;12(2):1157-1171.
22. Kohnen T, Lapid-Gortzak R, Ramamurthy D, et al. Clinical outcomes after bilateral implantation of a diffractive trifocal intraocular lens: a worldwide pooled analysis of prospective clinical investigations. Clin Ophthalmol. 2023;17:155–163.
23. Lee YW, Choi CY, Moon K, et al. Clinical outcomes of new multifocal intraocular lenses with hydroxyethyl methacrylate and comparative results of contrast sensitivity, objective scatter, and subjective photic phenomena. BMC Ophthalmol. 2022;22(1):379.
24. Kinoshita K, Miyata K, Nejima R, Honbo M, Mori Y, Minami K. Surface light scattering from 1-piece hydrophobic acrylic intraocular lenses with hydroxyethyl methacrylate: contralateral observation for 7 years. J Cataract Refract Surg. 2021;47(6):702-705.
25. Rementeria et al. Visual performance and patient satisfaction outcomes of non-diffractive EDOF IOL in a large real world cohort after cataract surgery. Presented at the American Society of Cataract and Refractive Surgery (ASCRS) Annual Meeting: May 5-8, 2023; San Diego, CA.
26. Sohee J, Choi A,Kwon H. Clinical outcomes after implantation of extended depth-of-focus AcrySof® Vivity® IOL in eyes with low-grade epiretinal membrane. Presented at the Asia-Pacific Association of Cataract & Refractive Surgeons (APACRS); June 8-10, 2023; Suntec City, Singapore, 2023.
© Alcon Inc. 10/23 US-CLM-2300024






