Dry eye disease (DED) is one of the conditions eye care professionals encounter most often. Although millions of patients have been diagnosed with DED, far more remain undiagnosed. With many new treatments and diagnostic tools available, it can be difficult for a clinician to know where and how to start when launching a dry eye clinic. This article provides some guidance.
WHY DIAGNOSE DED?
Not so many years ago, clinicians generally viewed DED as a nuisance experienced by a minority of patients, and treatment options were limited. Managing the condition involved significant chair time, and patients often remained dissatisfied. Thanks to a growing body of research, attitudes about DED as well as options for its diagnosis and treatment have changed.
The Prospective Health Assessment in Cataract Surgery Patients’ Ocular Surface (PHACO) study evaluated individuals scheduled for cataract surgery. The study found that, although only approximately 20% of the patients had been previously diagnosed with DED, 77% of the 136 enrolled patients had corneal staining, and 50% had central corneal staining. In addition, 63% of the patients had a tear breakup time (TBUT) of 5 seconds or less, and 18% had a Schirmer score with anesthesia of 5 mm or less.
The PHACO study suggests that DED is prevalent yet underdiagnosed, but why should eye care providers care if most patients with the condition—particularly those undergoing cataract surgery—are not complaining? The answer is that DED affects surgical outcomes. I typically explain to my patients that ocular surface disease must be treated before cataract surgery for three reasons:
- No. 1: To prevent infection;
- No. 2: To facilitate accurate preoperative measurements and IOL calculations; and
- No. 3: To prevent postoperative aberrations.
Even if the IOL power calculation is correct, an unhealthy ocular surface can compromise a patient’s postoperative vision. This concern is amplified when a multifocal or extended depth of focus IOL is implanted.
The concept of optimizing the ocular surface before cataract and refractive surgery has become more accepted in recent years, but DED warrants consideration with nonsurgical patients as well. Many individuals who have difficulty tolerating their contact lenses or who are unhappy with their vision have no pathology other than DED.
STARTING POINT: THE PATIENT HISTORY
The most important diagnostic tool in my clinic is the patient history. Many practices use a standardized patient survey, several of which have been validated in the literature. These surveys work well but may not be necessary. A targeted history obtained by asking pointed questions can suffice.
It is not enough to ask a patient if they have dry eyes. Epiphora is one of the most common complaints I hear from individuals who do not realize that they have DED. These patients often reject the diagnosis initially because DED in the presence of excessive tearing seems counterintuitive.
Fluctuating vision can also be a symptom of DED. I often encounter patients who, during the cataract surgery evaluation, say they have difficulty reading. Upon further questioning, they may clarify that they can read clearly for a period of time but then their vision gradually blurs, requiring them to take a break. I explain that cataracts do not fluctuate by the minute and their experience is more likely due to a poor-quality tear film.
THE EXAMINATION
TBUT and corneal staining. It is not essential to buy expensive equipment immediately upon starting a dry eye practice. In my opinion, vital dye staining is the most important diagnostic tool after the patient history. All my dry eye evaluations include fluorescein staining. Rather than use the solution for applanation, which contains an anesthetic, I place a fluorescein strip with a drop of saline in the fornix without touching the cornea or bulbar conjunctiva. This strategy allows me to assess the TBUT and corneal staining. Devices are available for automated, objective TBUT testing, but they usually are not necessary in a beginner clinic.
The literature contains several validated grading scales for the staining assessment, such as the National Eye Institute scale and the Oxford scale. I recommend selecting one and keeping a laminated card in the exam lane to promote consistency between visits and examiners.
I also stain the conjunctiva with lissamine green dye. The staining patterns demonstrated with fluorescein and lissamine green dyes may help me make a diagnosis, determine the etiology of DED, and grade its severity (Figures 1 and 2).1
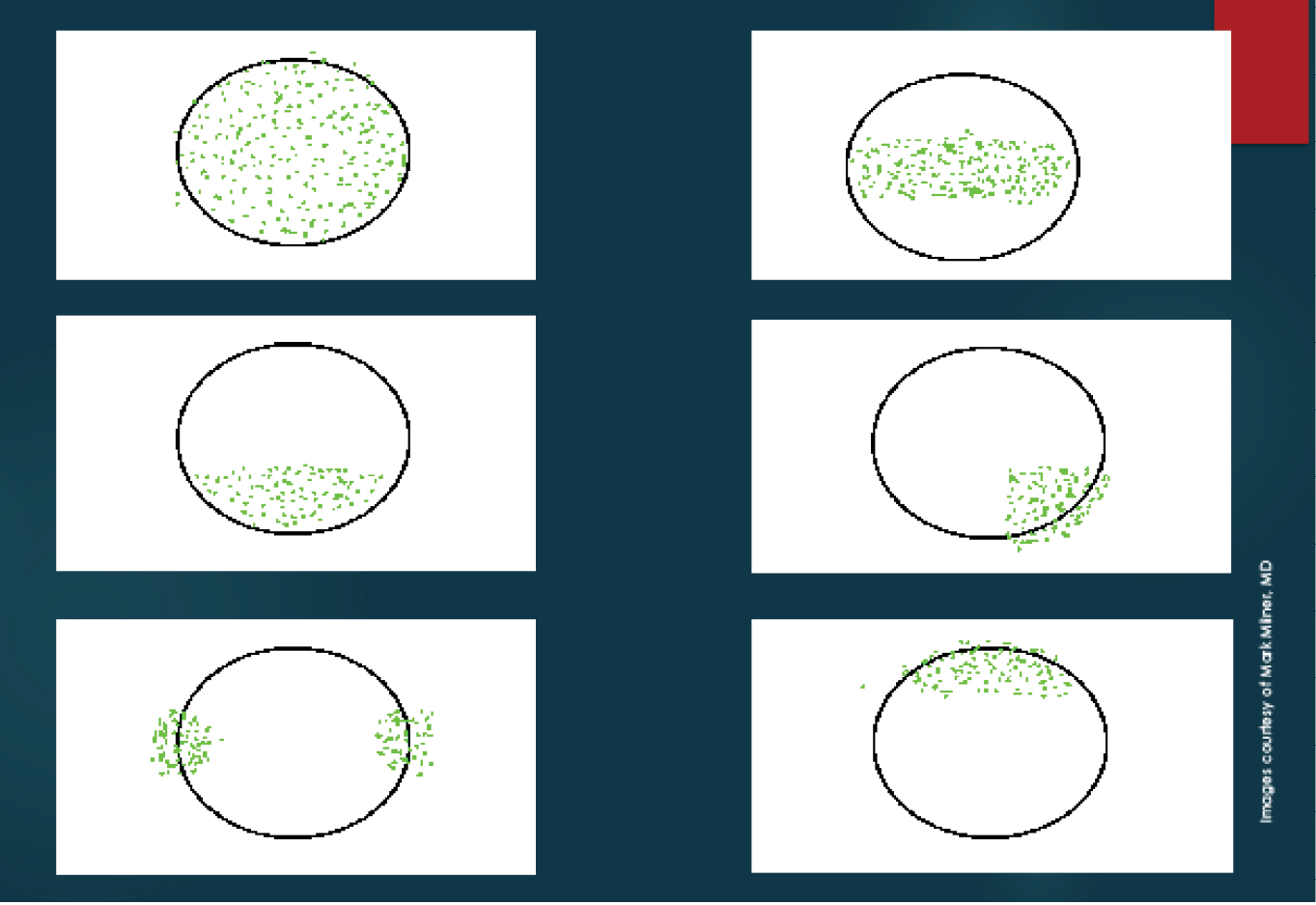
Figure 1. Dysfunctional Tear Film panel’s approach to ocular surface fluorescein staining patterns.2 Severe keratoconjunctivitis sicca (A), moderate keratoconjunctivitis sicca (B), blepharitis or exposure keratopathy (C), medication toxicity (D), contact lens–related keratitis (E), and superior limbic keratitis (F).
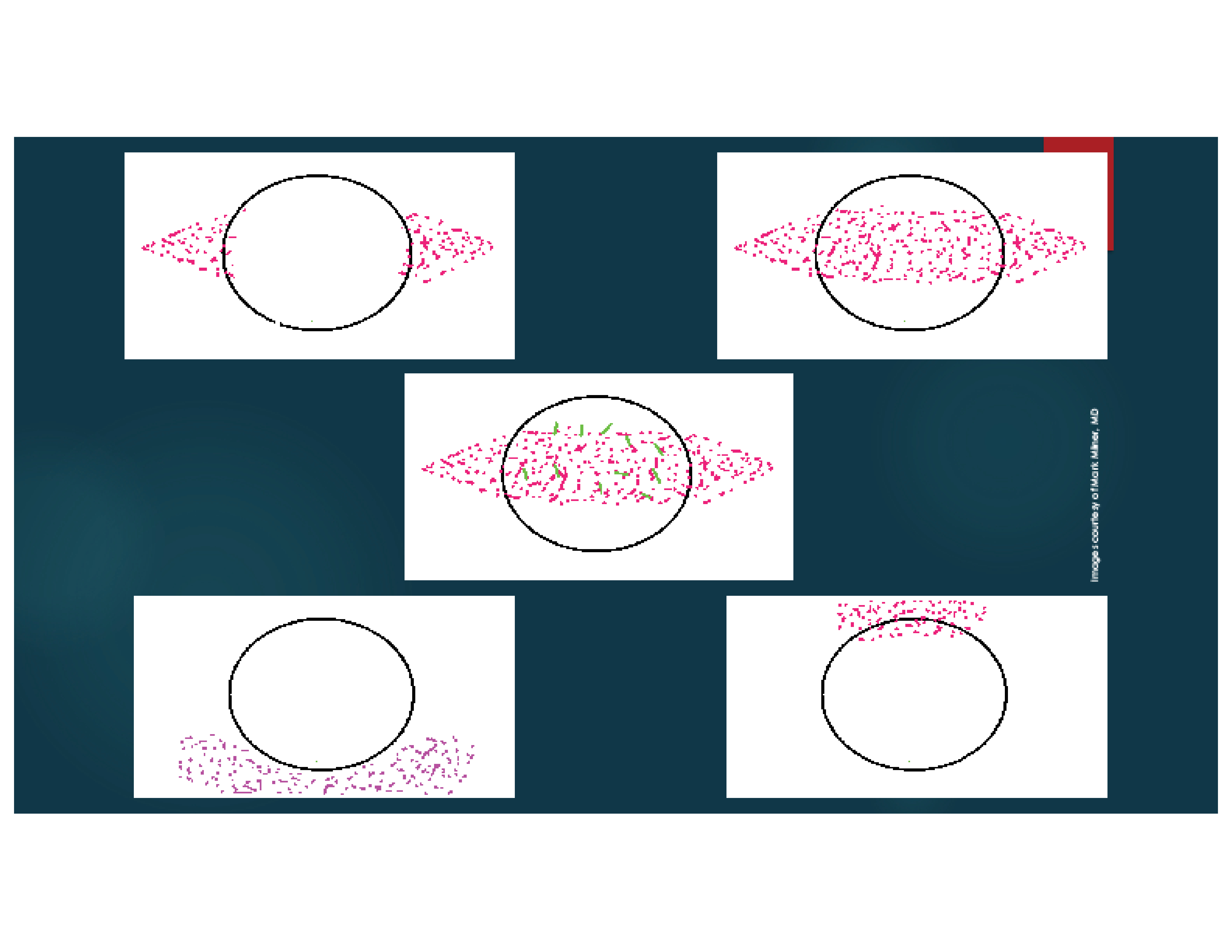
Figure 2. Dysfunctional Tear Film panel’s approach to ocular surface lissamine green or rose bengal staining patterns.2 Mild keratoconjunctivitis sicca (A), moderate keratoconjunctivitis sicca (B), severe keratoconjunctivitis sicca (C), mucus fishing syndrome (D), and superior limbic keratoconjunctivitis (E).
Lid evaluation. Another inexpensive, readily available diagnostic tool is a thorough lid evaluation. An assessment of the entire lid—including the lid margin, lash line, lid closure, blink rate and completeness, and meibomian expression—can provide a tremendous amount of information. Pushing on the lid margins can rapidly indicate how they are functioning.
Imaging of the meibomian glands can provide insight into their viability. Several devices are available for this purpose. When starting a dry eye clinic, however, simple eversion and retroillumination of the lids with a muscle light can allow an assessment of meibomian gland structure.
Tear osmolarity and inflammation. An observational, prospective, nonrandomized study found that patients with high tear osmolarity (> 316 mOsm/L) during preoperative testing for cataract surgery had statistically significantly greater variability in average keratometry readings and anterior corneal astigmatism than those with normal osmolarity.3 This led to significant differences in IOL power calculations.
I regularly measure patients’ tear film osmolarity with the TearLab Osmolarity System (Trukera Medical) to diagnose DED, grade disease severity, and assess patients’ responses to treatment over time.
The concentration of the inflammatory marker matrix metalloproteinase-9 (MMP-9) is often elevated in dry eyes. In my practice, MMP-9 testing occurs at the initial visit or at an early visit. The presence of significant inflammation may prompt me to pursue more aggressive antiinflammatory treatment in lieu of placing a punctal plug.
Both tear film osmolarity and MMP-9 testing should be performed at the beginning of the examination, before any topical drops are administered.
Additional tests. Keratometers and topographers can be used to identify DED. Patients with seemingly normal exam findings who report experiencing blurry vision may have abnormal topographic findings or mires on keratometry that indicate a poor-quality tear film.
Additionally, the tear meniscus height can be measured with the light beam of the slit lamp.
CONCLUSION
Many devices are available for DED diagnosis. Practitioners and practices new to this area of eye care may wish to avoid a substantial capital investment in equipment at the outset. Fortunately, there are many inexpensive ways to assess the tear film. With simple approaches such as a thorough patient history, a detailed slit-lamp examination, and the use of vital dyes, clinicians can quickly build a dry eye practice and then, when they are ready, expand it with other diagnostic devices and treatment modalities.
1. Trattler WB, Majmudar PA, Donnenfeld ED, McDonald MB, Stonecipher KG, Goldberg DF. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423-1430.
2. Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders – new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27 Suppl 1(suppl 1):3-47.
3. Epitropoulos AT, Matossian C, Berdy GJ, Malhotra RP, Potvin R. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg. 2015;41(8):1672-1677.




