
Dr. Singh invited Dr. Seibold to talk about new and emerging options in the MIGS space: one targeting outflow in and beyond the trabecular meshwork (TM), and a new take on directing outflow to the subconjunctival space.
What is the Streamline Surgical System?
The concept of flushing the TM, canal of Schlemm, and adjacent anatomy with OVD, or viscodilation, has grown in popularity in recent years because surgeons have a better understanding of how complex the aqueous drainage pathway actually is. Although the TM is the most typical site of obstruction in primary-open glaucoma, blockades at other sites may inhibit outflow. Viscodilation provides a means to open up herniations and blockages, even without knowing the exact location of collector channels.
Dr. Seibold has been using the Streamline Surgical System (New World Medical, Inc.) since its FDA approval more than a year ago. The device is designed to perform a 150-µm goniotomy and deliver OVD in a single step, thereby opening a drainage pathway, stretching open the TM, and flushing the canal and distal collector channels. The treatment can be titrated, as it can be performed up to eight times across the nasal angle. As well, the surgeon can extend the goniotomies by using the cannula to create a linear goniotomy for a few clock hours.
These features add tremendous versatility, yet the device is also very minimally traumatic to the ocular tissues, according to Dr. Seibold. Early evidence suggests a role for the procedure in treating mild and early glaucoma, although recent evidence has demonstrated a benefit in more advanced cases.
Lazcano-Gomez and colleagues recently reported an interim analysis of an ongoing prospective, nonrandomized, open-label, interventional, first-in-human case series to characterize the safety and IOP-lowering effectiveness of incisional goniotomies and canal of Schlemm viscodilation in patients with mild to severe POAG undergoing cataract surgery.”1 At the 6-month readout, about 90% of patients achieved more than a 20% reduction in IOP, and over 40% were free of medication (Figure 1).
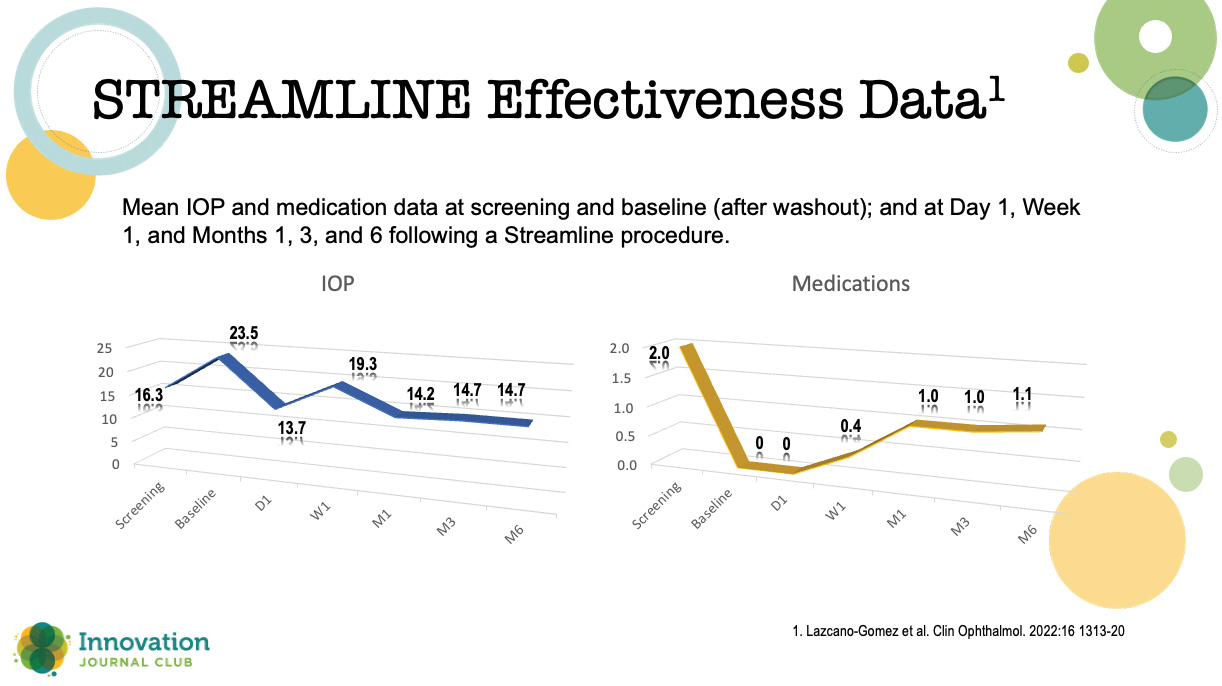
Figure 1. Results from an interim analysis of an ongoing prospective, nonrandomized, open-label, interventional, first-in-human case
“The mean medication use fell in half,” Dr. Seibold said, “with 40% or a little bit more achieving medication-free status. This is really important. These are the home runs we want to hit with our patients with MIGS.”
Minimally Invasive Micro Sclerostomy
When it comes to unguarded filtration surgery, glaucoma surgeons would not be wholly unjustified in responding with a “been there, done that” mindset. However, minimally invasive micro sclerostomy (MIMS), being developed by Sanoculis, Ltd., because it is performed via an ab interno approach, may be a viable option for effecting subconjunctival drainage without leaving an implant in place and without opening conjunctiva like traditional incisional glaucoma surgeries.
“With MIMS, we also don’t have to worry about implant placement,” Dr. Seibold said.
The surgery is performed with a disposable handpiece that creates a precise and repeatable 100-µm microsclerotomy from an ab interno approach. Then, the surgeon advances a small trephine into the sclera to extract a plug of tissue and create a fistula pathway into the subconjunctival space. The energy delivery is controlled via a footpedal.
Geffen et al published results of a prospective, open-label clinical trial of a MIMS procedure performed by a single surgeon in Chennai, India (Figure 2).2 Adults with uncontrolled open-angle glaucoma underwent either phacoemulsification plus MIMS (n = 21) or MIMS alone (n = 10). The criteria for success included a post-procedure IOP ≥ 5 mm Hg and ≤ 18 mm Hg; a reduction in IOP of > 20% compared with baseline; and no need for filtration surgery at 12 and 24 weeks after surgery.
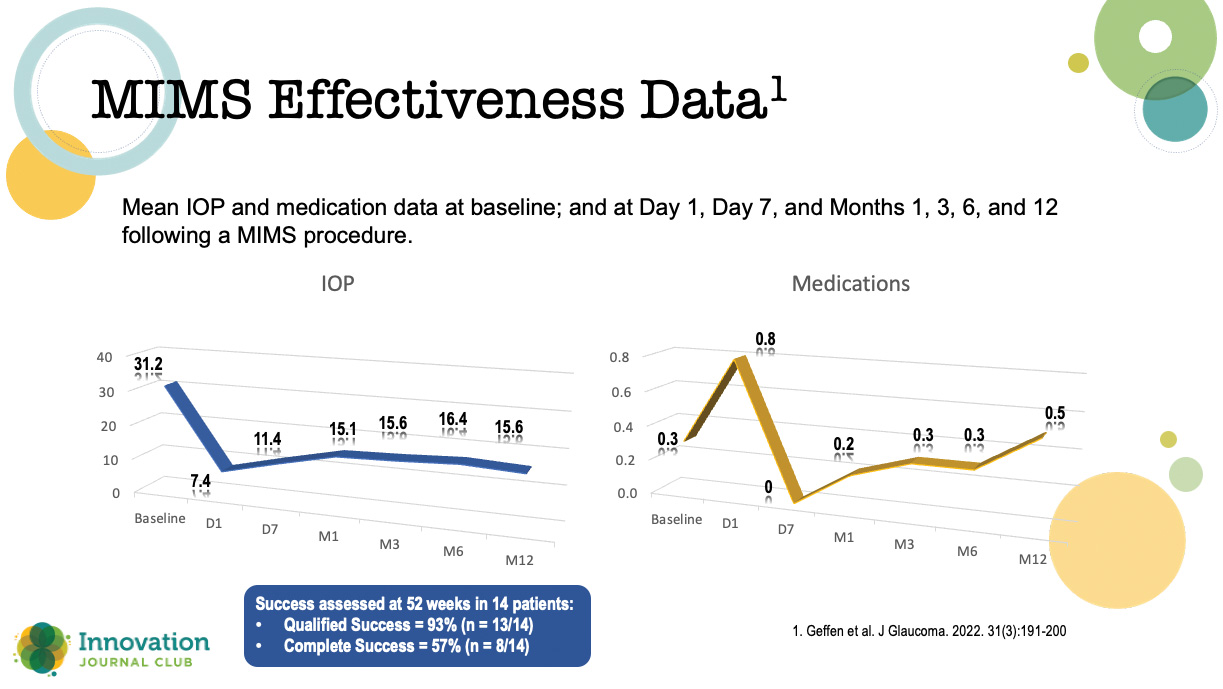
Figure 2. Results from a prospective, open-label clinical trial of patients undergoing MIMS or MIMS plus cataract surgery.
The authors reported no intraoperative complications, serious adverse events, or device malfunctions; however, five patients (16.12%) experienced iris clogging of the internal ostium between 1 and 24 weeks following the procedure: two were treated with a laser iris retraction and thereafter maintained a stable IOP, and three required a trabeculectomy and were considered surgical failures.
“I think there was a little bit of AC shallowing, and that allowed the iris to adhere to that sclerotomy and occlude it,” Dr. Seibold said. “[The authors] presented a number of different ways to prevent this, [including] some good suggestions on using a miotic agent like pilocarpine and leaving viscoelastic in the eye, which I think makes a lot of sense.”
Although he does not have personal experience with the procedure, Dr. Seibold said he will be monitoring clinical trials with the technique for answers to some lingering questions: “How do these patients behave early postoperatively? Can we leave a little viscoelastic? I think we can manage those things, and this could potentially be a nice option for patients who maybe want to stay away from a stent … [or who] have thin conjunctiva. I think this would give us a nice alternative.”
1. Lazcano-Gomez G, Garg SJ, Yeu E, Kahook MY. Interim analysis of STREAMLINE® Surgical System clinical outcomes in eyes with glaucoma. Clin Ophthalmol. 2022;16(4):1313-1320.
2. Geffen N, Kumar DA, Barayev E, et al. Minimally invasive micro sclerostomy (MIMS) procedure: a novel glaucoma filtration procedure. J Glaucoma. 2021;31(3):191-200.
