Although initially conceived to treat inherited retina disease, gene therapy is now being studied as a novel approach to manage common retinal pathologies like the dry and wet forms of age-related macular degeneration (AMD) and diabetic retinopathy (DR). In an episode dedicated to the state of the science around gene therapy in ophthalmology, Dr. Singh interviewed David Eichenbaum, MD, to learn about the different types of gene therapy, how they work, and why there is so much excitement for its potential.
Addressing Unmet Need: The Burden of Care Associated
with Anti-VEGF Therapy
According to Dr. Eichenbaum, the current research focus in gene therapy may be for common retinal diseases, but it has potential to impact all of ophthalmology’s subspecialties: “Gene therapy, even though it's focused on retina right now, has the potential to affect glaucoma, cornea—everything that we do, and it's in its early days.”
He explained that the form of gene therapy primarily being explored for retinal disease is gene augmentation (Figure 1). “In the case of most of our common retinal disease gene therapy programs, we’re directing the cell to produce a therapeutic protein, a ranibizumab-like product,” he told Dr. Singh. The therapeutic goal is to reduce (or eliminate) patients’ dependence on medications or injections, and thereby increase quality of life, to say nothing of boosting compliance and protecting the health of the ocular tissues (Figure 2).
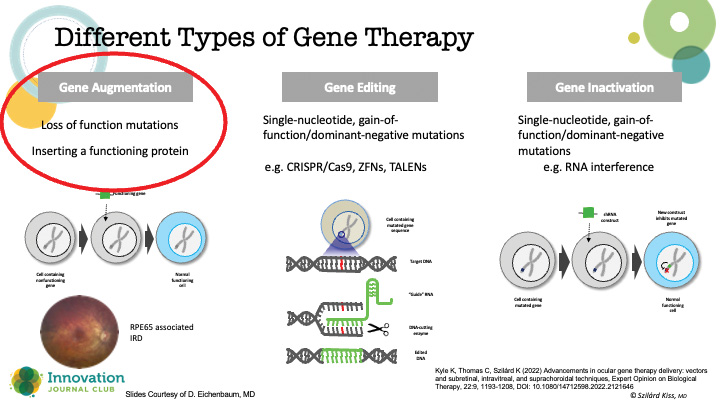
Figure 1. Various types of gene therapy.

Figure 2. Proponents hope that gene therapy can be a “one and done” option for addressing common retinal pathologies like AMD and DR.
But why has ophthalmology attracted so much attention for gene therapy research? Dr. Eichenbaum explained that the eye has several attributes that make it the perfect testing ground for gene therapy: It is small, easily accessible, is receptive to many forms of imaging technologies, can be examined directly and monitored for both therapeutic and adverse effects, and, most importantly, it self-contains any adverse reactions. As well, until recently, the only FDA-approved gene therapy across all of medicine was for RPE65-mediated retinal dystrophy (voretigene neparvovec; LUXTURNA, Spark Therapeutics). The latter demonstrated the viability of using viral vectors to deliver genetic material to retinal cells, thus serving as a starting point for investigations into the treatment of common retinal diseases.
How Gene Therapy Is Delivered to the Retina
Dr. Eichenbaum explained that gene therapy is essentially the use of a viral vector that contains genetic material that, when introduced into the nucleus of cells, reprograms their behavior (Figure 3). “In ophthalmology, we're using adenoviral-associated vectors because they have a low risk of genotoxicity, they can carry a large genetic payload, and they’re fairly well tolerated with efficient transduction,” he said. Importantly, there are ways to test if the changes to the DNA are having the desired effect on the disease.
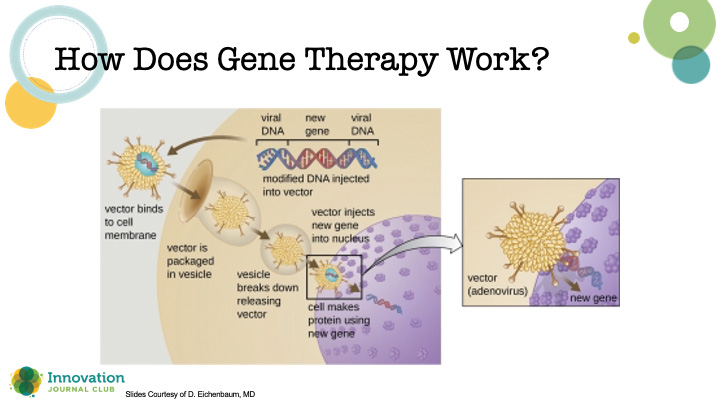
Figure 3. Schematic depicting the crucial steps for introducing genetic material to a host cell to induce a therapeutic effect.
There are three methods for delivering gene therapy currently under investigation. First is what Dr. Eichenbaum called the aspirational approach: intravitreal. This would be the preferred method of delivery since retina specialists are already well-versed in the technique. However, this method appears to have barriers, such as requiring a higher dose. As well, in early studies, there appears to be a higher rate of inflammation than other delivery methods (perhaps related to dosing), and researchers have noted physical barriers to delivering the product to the cells, like the inner limiting membrane.
Another method researchers are using is subretinal delivery, “because that seems to be the safest place to put it with regards to inflammation,” said Dr. Eichenbaum. One example of subretinal delivery is ABBV-RGX-314 (AbbVie/Allergan and Regenxbio), which is currently being investigated in a phase 3 study for wet AMD.
A third option for gene therapy delivery is suprachoroidal injection. This approach is in use in the phase 2 trial of ABBV-RGX-314 for treating DR, and Dr. Eichenbaum said that it may have potential down the line for treating macular degeneration. Its drawbacks, he said, are that the technique is “a little trickier and a little less routine than intravitreal injections,” and that the suprachoroidal injections are showing more inflammation in the phase 2 trial compared with subretinal injections. Yet, he feels that these are surmountable challenges.
Current Research Focus and Patient Selection
Per Dr. Eichenbaum, most of the current research into ophthalmic gene therapies is focused on common retinal diseases that require injections, such as wet AMD, geographic atrophy, and DR, and for the most severe cases thereof—the patients who will benefit the most. The clinical trials of these products will answer the necessary questions of efficacy and safety, length and duration of effect, and whether they eliminate or just reduce the burden of injections. As part of these studies, researchers will also examine the best ways to mitigate the inflammatory response of gene therapy. He pointed out that as the ophthalmic population continues to age, these diseases will become more common, and thus the potential demand for gene therapy could be great.

