INTRODUCTION
Sheri Rowen, MD: Dry eye disease (DED) affects countless individuals, most often arising from excessive tear evaporation exacerbated by meibomian gland dysfunction (MGD).1-6 The resultant instability of the tear film leads to heightened ocular surface desiccation and inflammation.
In May, the FDA approved MIEBO (perfluorohexyloctane ophthalmic solution, Bausch + Lomb), the first and only prescription DED eye drop that directly targets tear evaporation. MIEBO is different from existing prescription approaches, which include immunomodulators, tear stimulators, and antiinflammatory agents. This single-ingredient drop consists of 100% active ingredient, is water-free, preservative-free, and steroid-free, and is designed to inhibit tear evaporation at the ocular surface to mitigate the adverse effects of evaporative DED.
The availability of MIEBO marks a significant stride toward addressing an unmet need for millions of individuals suffering with the pervasive condition of DED, and quite frankly is a turning point in evaporative DED management. All of us participating in this roundtable are early adopters of MIEBO. Our discussion here illustrates the need for MIEBO as a targeted evaporative DED treatment option, reviews clinical data, and suggests treatment protocols in targeted patient profiles.
Studies have indicated that 86% to 92% of DED patients have an evaporative component (Figure 1).1,2,7 We therefore can assume that almost every patient we see has an evaporative component. I have seen many patients with Sjögren syndrome and rheumatoid arthritis who classically I thought had purely aqueous deficient DED only to find out that they have significant MGD and evaporative DED as well.
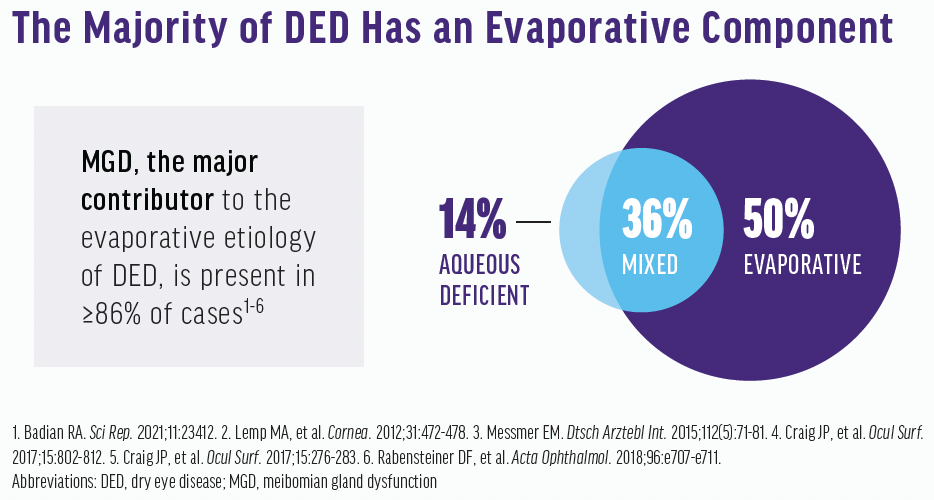
Figure 1. There is a high prevalence of evaporative DED.
James Katz, MD: The classic teaching was to diagnose and treat aqueous deficiency, but now we realize that more than 90% of individuals have mixed mechanism or purely evaporative DED. We need to address that better, and I think MIEBO allows us to do just that.
Dr. Rowen: Before MIEBO, has anyone felt they could deal with the evaporative component directly?
Laura M. Periman, MD: Not directly.
Brandon D. Ayres, MD: We’ve all come to realize that we don’t understand DED as well as we thought. There’s so much mixed mechanism disease. Some treatments target inflammation, but they don’t stabilize the tear film. I am excited to have a treatment that targets a component that we’ve never been able to prescribe a medication for before.
Dr. Periman: In real time. That’s what’s so exciting.
Marguerite B. McDonald, MD, FACS: You could say everything we’ve done until now has been indirect. MIEBO is a direct treatment for evaporative DED.
Dr. Periman: An unstable tear film, particularly in the form of evaporative stress, results in desiccating stress on the ocular surface. The system is primed to respond in an inflammatory way, creating tissue damage and resulting in more tear film instability (Figure 2).8,9 It’s a vicious downward spiral that needs to be addressed directly. If it’s ignored, it will continue to get worse.
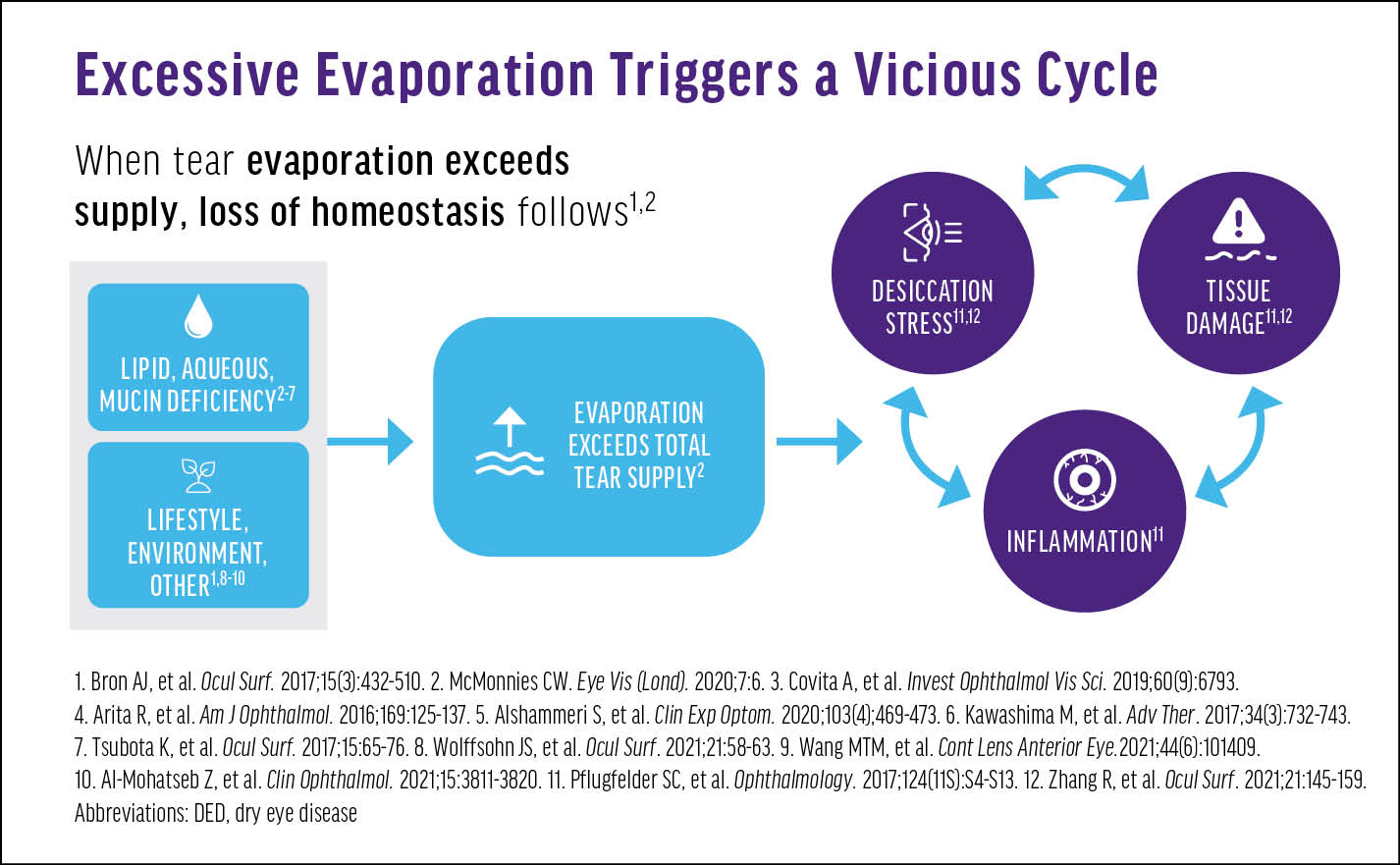
Figure 2. The downstream effects of excessive evaporation of tears.
Dr. Ayres: We’ve been asking patients to perform warm compresses and lid massage to treat the meibomian glands first. Now that we have a prescription eye drop that can directly target the evaporative component, maybe we can address the ocular surface disease (OSD) at a much earlier part of the cascade, preventing some of the downstream complications and inflammation.
Dr. Katz: I think that’s a great place to treat it because, again, we’re not just talking about lipid deficiency, but mucin deficiencies, environmental factors, and other external factors that also contribute to DED.
Dr. Rowen: The environmental component is crucial. There is a steep increase in the use of digital devices. When someone stares at a device, their blink rate decreases. Having something on the surface of the eye so that the tear film won’t evaporate too quickly while they’re not blinking could be a helpful adjunct.
Dr. McDonald: We tell patients about the 20/20/20 rule while at their computers—every 20 minutes, stand up for 20 seconds, and look 20 feet away—to get them to blink more often and more completely. Blinking spreads the tears evenly and helps to release meibum via a pumping action on the meibomian glands. I ask patients if they do it, and they admit they don’t. So, it doesn’t provide them much relief, but MIEBO does.
Dr. Rowen: In fact, the relief is remarkable. There may be minimal to no stinging or burning. Within a few seconds of a blink, most patients who have used it in my office describe it as a silky feeling. They don’t feel the friction anymore.
Dr. Ayres: I’ve had it in my own eyes. As reported in the clinical trials, it felt very comfortable.
Dr. Ayres: We don’t always see the impact that DED has on a patient’s life. By taking the disease seriously, we help patients function better in life. With other medication, I had to worry about if it would irritate them and how long it would take to work. With MIEBO, however, we can quickly say, “You are experiencing more than just dryness, and it is affecting your life in negative ways. This drop is expected to make your eyes feel better quickly, within about 2 weeks.”
Dr. Rowen: More than 38 million patients in the United States have DED10 but only about 18 million have been diagnosed11 and 1.2 million have been treated.12 Of those, the compliance rate is generally low. Patients might take the drops for several months if they get something out of it, but in my clinical experience discontinuation rates are high with common DED medications.
Now that we have an option that is comfortable on instillation and can help them feel better after 2 weeks, I think we’ll see fewer dropouts.
Dr. Ayres: Patients are often dissatisfied with the slow treatments that we have been using for years. It’s nice to have a product that we can stand behind.
Dr. Katz: MIEBO may be used to improve patient symptoms over the long term. Not only may the drops slow progression of the disease with continued use, but patients’ symptoms may be much improved.
Dr. Rowen: I agree, and once they’re being treated, then we can address any residual inflammation. MIEBO is complementary to other products.
Dr. Periman: I see MIEBO as a first-line option to stabilize the tear film while we work on the machinery that contributes to a healthy ocular surface. I can consider it being used in conjunction with other therapies. Some of these may include in-office strategies to coax the meibomian glands to function better; the meibomian glands are no longer being constantly taxed by an evaporative load and desiccating stress, which we know fuels the fire of MGD.
Dr. Ayres: I think this medication is going to be prescribed at the starting gate for a lot of patients, while other medications can be prescribed to fight the inflammatory response and increase aqueous production.
Dr. Periman: DED is a chronic and progressive disease that, untreated, is a vicious downward cycle.
Dr. Rowen: How do we identify these patients accordingly? What strategies does everyone use?
Dr. Katz: Symptomatology questionnaires can be helpful to find out about patients’ visual fluctuation, blur, and eye fatigue—symptoms they don’t normally recognize. The eye exam is equally important. Dyes can be used to identify signs of DED and/or evaporative disease.
MARGUERITE MCDONALD, MD, FACS
A 27-year-old woman presents for a LASIK evaluation. She works as a spa manager. She takes birth control pills, drinks alcohol socially, and occasionally sleeps with eye makeup on and with her contact lenses in. Her chief complaints are difficulty wearing contact lenses for more than 6 hours without discomfort and eye redness. She wears daily disposables when she can, and she’s unhappy wearing glasses. She has self-treated with many over-the-counter artificial tears and found them to be ineffective.
On examination, mild to moderate injection and inspissated glands are found in the right and left eyes. Also in both eyes, the tear breakup time is 4 seconds, there is presence of grade 2+ superficial punctate keratopathy, the BCVA is 20/25, and the speed score is 8. On meibography, moderate gland truncation and dropout are noted.
The patient was diagnosed with evaporative DED due to MGD with a component of aqueous-deficient dry eye due to her use of birth control pills. Her dry eye was exacerbated by her lifestyle.
Initial treatment included warm compresses, eyelid hygiene, and over-the-counter drops. On follow-up examinations, she reported minimal improvement and was prescribed an antiinflammatory drop, which she discontinued after 6 days due to burning with instillation. She continued to complain of eye dryness symptoms.
Because it was likely that her evaporative dry eye with an aqueous deficient component was multifactorial and the ocular surface needed to be stabilized before her LASIK preoperative evaluation, MIEBO was the right choice for treatment. Patients should be counseled that they must remove their contact lenses before instillation and leave them out for 30 minutes after dosing.
There are a lot of women just like this patient who would be good candidates for MIEBO. As a sidebar, most female dry eye patients who report not taking drops during the day said they didn’t want the drops to roll down their face and smear their makeup. The small drop size of MIEBO makes it much less likely to roll down the patient’s face.
Dr. Rowen: The signs and symptoms of DED don’t always correlate. Some patients come in to our practices having significant symptoms but nothing on their surface, and others come in having no symptoms but a damaged ocular surface. In our diagnostic approach, we must look for both components.
Dr. Periman: Now with MIEBO, we have an excellent opportunity to intervene early.
Dr. Ayres: Some clinicians do not screen for DED because they haven’t had a positive experience with the available treatments. I encourage those individuals to give MIEBO a try because it may give them a positive experience. Once you can help a patient with DED, you’ll become more invested and you may even try other procedures.
Dr. Rowen: In many cases, I have been using MIEBO as a first-line treatment with great success.
Dr. McDonald: Studies have shown that diagnosing and treating DED preoperatively helps surgical outcomes and facilitates patients’ faster visual recovery. It optimizes the ocular surface and provides the best opportunity to achieve accurate preoperative biometry measurements. Prescribing MIEBO is a game-changer. You can say, “Here, take this drop four times a day and come back in 2 weeks, and we’ll do your preoperative evaluation for LASIK.”
Dr. Katz: I love looking at this from a workflow perspective. I expect that the low side effect rate of MIEBO may result in limited callbacks.
MECHANISM OF ACTION
Dr. Rowen: Laura, can you discuss the mechanism of action and how MIEBO inhibits evaporation?
Dr. Periman: The perfluorohexyloctane molecule is an amphiphile; it has two distinct segments, one which is aerophilic and one which is lipophilic. Perfluorohexyloctane also has a low surface tension and spreads rapidly to form a monolayer on the surface of the tear film after instillation. The fluorinated end of perfluorohexyloctane sticks into the air, and the hydrocarbon tail, the hexyloctane portion, integrates with the lipid component of the tear film. Perfluorohexyloctane has unique properties because it is a semifluorinated alkane and its low surface tension means that the drop size is of low volume (11 µL).
The other interesting part is, unlike an artificial tear, which typically has a residence time of 5 to 10 minutes, nonclinical studies have shown that perfluorohexyloctane can last up to 6 hours in tears.13,* It also inhibited the evaporation of saline four times more effectively in an in vitro study than meibum lipids from a healthy volunteer, suggesting it’s more efficient in preventing evaporation than natural meibum as well.14,*,†
Dr. Katz: Essentially, MIEBO forms a monolayer at the air-tear interface, which can be expected to reduce evaporation.
Dr. Periman: Well summarized.
Dr. Rowen: It also may reduce friction, so patients don’t feel their eyelids rubbing against their eyeball.
Dr. Periman: I like to sample dose one eye and at different time points ask, “Which eye feels better?” That helps illustrate the benefit of addressing the evaporative load with this molecule.
Dr. Rowen: Also, because there is no water in MIEBO, it does not need a preservative.
Dr. Ayres: It is a unique thing about this medicine. We’ve never had a product before that is comprised of 100% active ingredient.
Dr. Rowen: Most eye drops are instilled from the bottle in about a 35- to 50-µL dose, but after tearing and blinking the majority of the dose is washed away. MIEBO is designed as an 11-µL drop. It’s exactly what you need to coat the eye. Individuals might feel an oily layer on their lower lid when it drops in, but it doesn’t tear down the face. Most patients didn’t report any visual disturbances on instillation. In the GOBI and MOJAVE clinical trials, there was a very low incidence of blurred vision upon instillation, and it was not a cause for discontinuation.15,16
Dr. Periman: It’s satisfying to watch the tear film wet and MIEBO spread across the ocular surface after instillation. Instead of being clumpy and chunky, it’s smooth and evenly distributed. This is probably because of the uniform tear distribution that can be seen under fluorescein at the slit lamp. We treat a lot of patients with deltas in the height of the ocular surface, and the elevated regions desiccate first. MIEBO helps ensure even distribution of the tear film, helping prevent evaporation on the elevated spots.
Laura M. Periman, MD
A 49-year-old woman with longstanding advanced Sjögren syndrome with multiorgan system involvement presented with severe DED about 4 years ago.
During this time, multiple ophthalmic and systemic prescription medications and in-office procedures were performed. She experienced good improvement in the signs and symptoms of her DED, but the keratitis and symptoms persisted.
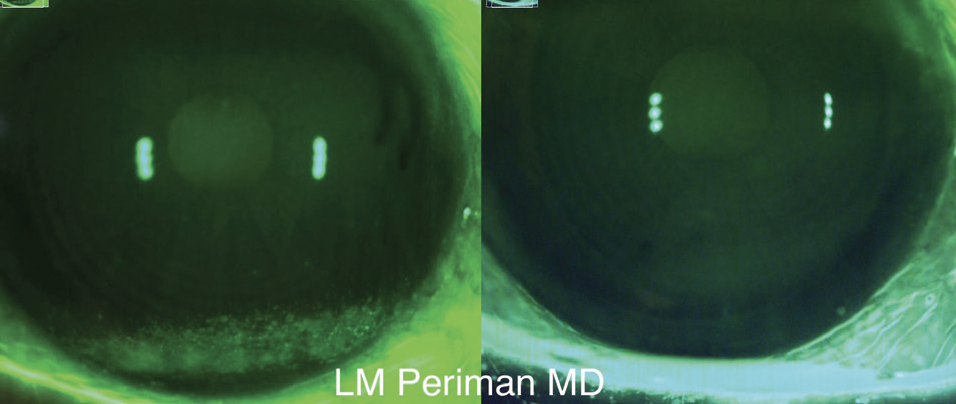
Figure. The patient’s eye before (left) and after (right) MIEBO QID for 2 months.
With the addition of MIEBO four times a day, in 2 months there was an appreciable improvement in her keratitis and symptoms (Figure). She has been able to decrease her systemic medications and reduce her topical steroid therapy to once a day. Her DED signs and symptoms have improved, which is wonderful to see. Treating the evaporative component directly with MIEBO provided her with what was needed to experience the needed additional improvements over a relatively short period of time.
SAFETY AND EFFICACY
Dr. Rowen: We know how MIEBO works, but let’s talk about the safety and efficacy profile. In two pivotal phase 3 clinical trials (GOBI and MOJAVE) involving more than 1,200 patients (more than 600 of whom were treated with MIEBO), MIEBO consistently demonstrated safety and efficacy in addressing the clinical signs and patient-reported symptoms of DED. Both studies met their primary endpoints. Across both studies, there were zero serious adverse events and a 0.2% rate of discontinuation due to ocular adverse events, which is extremely low. About 2.1% of patients reported blurred vision, mostly mild and transient. Eye redness was reported in 0.8% of patients, and burning and stinging on instillation was present in 0.5%.
Dr. McDonald: For my patients who wear contact lenses, I instruct my patients to remove their contact lenses before using MIEBO, and to wait 30 minutes before reinserting them.
Dr. Rowen: Brandon, can you discuss MIEBO’s efficacy data?
Dr. Ayres: You can usually get great effect but low tolerability or great tolerability but no effect. With MIEBO, we’ve seen great effect and tolerability, which is exactly what we’re looking for and what patients are looking for.
Both GOBI and MOJAVE were multicenter, randomized, double-masked trials. All patients had at least 6 months of a self-reported history of DED and had clinical signs of MGD with a meibomian gland score of 3 or more on a scale of 0 to 15. Patients were randomized one-to-one to MIEBO versus saline as the comparator since there is no vehicle for MIEBO.
The primary outcomes were total corneal fluorescein staining and eye dryness at days 15 and 57. Patients with active blepharitis, contact lens wearers, those who had punctal plugs or any procedure for MGD, and those who were on other DED medications, steroids, topical NSAIDs, and glaucoma drops were excluded.
BRANDON D. AYRES, MD
MIEBO is the first prescription drop I can prescribe that specifically targets evaporation. Once the ocular surface starts to heal, then I can add a immunomodulator if needed.
A woman with glaucoma and rheumatoid arthritis presented with a chief complaint of eye dryness as a result of prostaglandin analog use. After multiple intense pulsed light sessions, the tear film remained unstable, and she was still very symptomatic. This is exactly the kind of person that could benefit from MIEBO.
Already at day 15, there was a statistically significant improvement in total corneal fluorescein staining, indicating how quickly this treatment can work (Figure 3A). At day 57, there was a twofold improvement over the control.
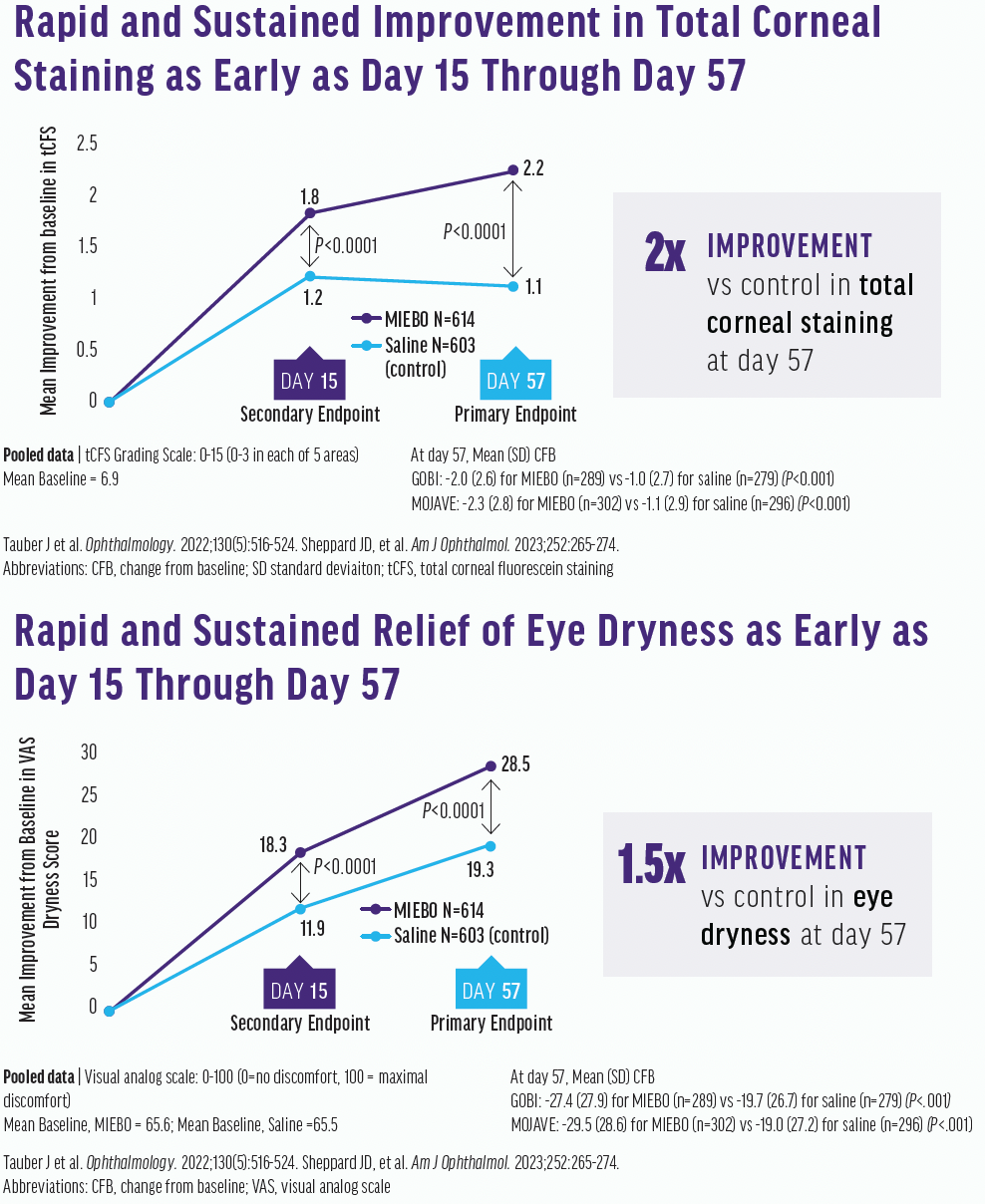
Figure 3. Improvements in total corneal fluorescein staining (top) and eye dryness (bottom) scores were seen at days 15 and 57.
The eye dryness score was done on a visual analog scale and graded from 0 to 100. It was typical for patients to improve from an eye dryness score of 66 to a mean of 37, showing effective relief of dry eye. At day 15, there was a statistically significant improvement over the control, which was maintained at day 57 (Figure 3B). Interestingly, the Kalahari study, which was an extension study looking at safety, showed a sustained effect through 12 months.
Dr. McDonald: This is so important. Preoperative biometry measurements for LASIK and cataract surgery are ruined by central superficial punctate keratopathy. These results show that, in 2 weeks, patients can experience clinical improvement. The speed with which MIEBO works to help get patients ready for surgery, to me, is invaluable.
Dr. Rowen: Not only do we get them ready for surgery, their signs and symptoms of DED will be improved prior to surgery. All of these put together with low adverse events, excellent tolerability, and a statistically significant improvement in corneal staining and eye dryness scores makes MIEBO a new agent that should be welcomed into all practices. There is no reason why anyone who has a diagnosis shouldn’t be started on it right away when they’re first seen.
MASTERING THE PATIENT CONVERSATION
Dr. Rowen: Each of us has prepared a case study for a certain patient profile that is appropriate for MIEBO (see the accompanying sidebars). Additionally, how do you counsel these patients about DED treatment, especially those who didn’t even know they had it?
Dr. McDonald: Backing up a step, a lot of patients in the baby boomer generation are still wearing contacts, so you must make sure they understand they have to discontinue contact use for a while before their preoperative evaluation. When they come in, they undergo tear osmolarity, the SPEED questionnaire, and topography. If they have symptoms on the SPEED questionnaire or a high tear osmolarity or rapid tear breakup time as seen on topography, the preoperative examination is stopped before any further diagnostic tests are performed. I meet them, greet them, and explain that they have DED and need to be on some kind of therapeutic treatment to optimize their ocular surface. These patients aren’t thrilled about having to wait a couple months before they can have surgery. Now with MIEBO, they can come back in as early as 2 weeks to be reassessed for surgery.
Sheri Rowen, MD
A man with graft versus host disease presented with severe DED. He couldn’t tolerate anything in his eyes, even artificial tears. I put him on MIEBO, and he said it’s the only thing that has made him comfortable. Although bad cases such as this can be rare, they also can be so frustrating for not only the patient but the clinician as well. If MIEBO helps in severe cases, imagine what it can do for patients with mild symptoms.
A second case I’d like to share is a woman with alopecia and autoimmune disease. She could not tolerate immunomodulators but was miserable without treatment. I prescribed MIEBO in combination with treatments that target the inflammatory components of DED, and the signs and self-reported symptoms improved significantly. Her ocular surface began to heal quickly, her vision was much better, and she was much happier.
These two case studies demonstrate the potential usefulness of MIEBO in outliers for whom we really haven’t had treatments before.
Dr. Rowen: When a patient’s topography and autorefraction don’t match, I immediately assess them for DED. In my practice, we like to get all our measurements upfront. Brandon, how do you work patients up initially? Do you do all your testing first, or do you stop it at a certain point?
Dr. Ayres: In an attempt for technicians to be efficient, they want to have everything ready for me. I often catch DED after everything is done. Maybe it took a little too much time, but we look closely for OSD. Truthfully, I love looking at topography because I pick up a lot of problems, and I can show them to patients so they can visualize the irregularities and better understand why they need DED treatment before surgery.
James Katz, MD
MIEBO is a great option to optimize the ocular surface before cataract surgery. In my practice, between 85% and 90% of patients who present for a cataract surgery evaluation have some signs or symptoms of DED, and in most of them there is an evaporative component.
Patients complete a symptomatology questionnaire, which includes questions like:
- Do your eyes feel dry?
- Do your eyes hurt?
- Do you experience eye redness?
- Do your eyes burn?
The answers drive the next step in their surgical journey, whether that be point of care testing, osmolarity testing, and/or meibography. Finally, the most important component is the clinical examination, where we look for common dry eye signs, including corneal staining and abnormal tear breakup time.
If a patient is diagnosed with ocular surface abnormalities, we discuss with them the process that is needed before biometry can be performed. In the past, patients who presented with any signs and/or symptoms of DED were prescribed artificial tears and asked to use warm compresses before they returned for a secondary assessment. Now, however, we can prescribe MIEBO because it addresses the evaporative tear component, and it does so quickly—in as little as 2 weeks. As a result, we don’t have to wait a month to reevaluate them. Typically, their symptoms improve rapidly after they start using MIEBO. In my opinion, this is one of the sweet spots for this product.
The faster we can get patients optimized and the fewer times we have to bring them back, the better it is for our schedule. The earlier a patient uses MIEBO, the faster we can get them into the OR.
Dr. Katz: Our number one job during the initial consultation is identifying DED patients with standard questionnaires, point-of-care testing, and even topography. You can see tear breakup on topography but can miss it on some other corneal measurements that use video over time. Looking at these measurements beforehand can alter how we treat our patients. MIEBO can get them better quickly so we can get an accurate biometry and better postoperative visual results.
Dr. Rowen: We should prepare our patients to understand that DED management is a process. It’s taken years for their ocular surface to degrade, and although it won’t take years to get back, we have to chip away using multiple modalities. They should be reminded to not get discouraged if improvements don’t happen overnight.
Dr. Ayres: Something that’s different when I talk to patients about MIEBO, however, is that I don’t have to prep them about many side effects. I simply tell them, “I have a medication that was shown to be very comfortable in the clinical studies for most people." Now, my discussion is so much shorter. There’s still a discussion, but patients have a more positive feel about what they may experience with that medication.
Dr. McDonald: Chair time adds up, so it really matters how many minutes it takes you to introduce a drug to a patient. When you tell them all the things that they might dislike, it sets a dreary tone. The less you need to explain a treatment and the more reasons you tell the patient it’s comfortable, so they may actually not feel it after instillation, the better.
Dr. Rowen: Exactly. The patient leaves a little happier, and we’re also happier. MIEBO should be positioned as that first-line drug for patients who present with evaporative DED. This was a great discussion, and I think we are all walking away with a couple pearls for patient counseling and selection.
*The clinical significance of these nonclinical data has not been established.
†Study design: The inhibitory effect of MIEBO vs meibum on the evaporation rate (Revap) of saline was evaluated in an in vitro model. Meibum lipids were collected from a single healthy volunteer. The Revap of saline was measured gravimetrically after layering either a single drop of MIEBO or the collected human meibum lipids to approximate the tear lipid layer in vivo over the top of 1 mL saline in a plastic container with a surface area approximating that of the human ocular surface. The Revap of saline was inhibited by 28% when a drop of MIEBO was layered over the top, while a layer of human meibum inhibited the Revap of saline by 8% (P < 0.0001 vs control for both). The clinical significance of this in vitro data has not been established.14
©2023 Bausch + Lomb MBO.0545.USA.23
1. Badian RA, Utheim TP, Chen X, et al. Meibomian gland dysfunction is highly prevalent among first-time visitors at a Norwegian dry eye specialist clinic. Sci Rep. 2021;11:23412.
2. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472-478.
3. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71-81.
4. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15:802-812.
5. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276-283.
6. Rabensteiner DF, Aminfar H, Boldin I, Schwantzer G, Horwath-Winter J. The prevalence of meibomian gland dysfunction, tear film and ocular surface parameters in an Austrian dry eye clinic population. Acta Ophthalmol. 2018;96:e707-e711.
7. Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151(5):792-798.e1.
8. Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology. 2017;124(11S):S4-S13.
9. Zhang R, Pandzic E, Park M, Wakefield D, Di Girolamo N. Inducing dry eye disease using a custom engineered desiccation system: impact on the ocular surface including keratin-14-positive limbal epithelial stem cells. Ocul Surf. 2021;21:145-159.
10. Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799-806.
11. 2020 dry eye products market report: a global analysis for 2019 to 2025. Market Scope. April 2021. Accessed August 28, 2023. market-scope.com/pages/reports/250/2020-ophthalmic-landscape-report-global-analysis-for-2019-to-2025-april-2021#reports.
12. IQVIA data, July 2021.
13. Krösser S, Spencer E, Grillenberger R, Struble CB, Eickhoff K. Ocular and systemic distribution of 14C-perfluorohexyloctane following topical ocular administration to rabbits. Invest Ophthalmol Vis Sci. 2018;59(9):2656.
14. Vittitow J, Kissling R, DeCory H, Borchman D. In vitro inhibition of evaporation with perfluorohexyloctane, an eye drop for dry eye disease. Curr Ther Res Clin Exp. 2023;98:100704.
15. Tauber J, Berdy GJ, Wirta DL, Krösser S, Vittitow JL; GOBI Study Group. NOV03 for dry eye disease associated with meibomian gland dysfunction: results of the randomized phase 3 GOBI study. Ophthalmology. 2023;130(5):516-524.
16. Sheppard JD, Kurata F, Epitropoulos AT, Krösser S, Vittitow JL; MOJAVE Study Group. NOV03 for signs and symptoms of dry eye disease associated with meibomian gland dysfunction: the randomized phase 3 MOJAVE study. Am J Ophthalmol. 2023;252:265-274.
INDICATION AND IMPORTANT SAFETY INFORMATION
Indication
MIEBO® (perfluorohexyloctane ophthalmic solution) is indicated for treatment of the signs and symptoms of dry eye disease.
IMPORTANT SAFETY INFORMATION
MIEBO should not be administered while wearing contact lenses. Contact lenses should be removed before use and for at least 30 minutes after administration of MIEBO.
- Instruct patients to instill one drop of MIEBO into each eye four times daily
- The safety and efficacy in pediatric patients below the age of 18 have not been established
- The most common ocular adverse reaction was blurred vision (1% to 3% of patients reported blurred vision and conjunctival redness)
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information for MIEBO here.








