PLU Ophthalmic was founded from a shared frustration among ophthalmologists over the persistent trade-offs in glaucoma surgery—choosing between efficacy and safety, or between complexity and consistency. Recognizing these unmet needs, glaucoma specialist Rodolfo A. Perez-Grossmann, MD, designed AquaLumen™ to provide a safe, implant-free, and reproducible alternative. Along with Carlos A. Urrea, MD, MPH, and myself, we combined his clinical innovation with decades of medtech leadership to bring this vision to life.
AQUALUMEN™
AquaLumen is a precision-engineered device that enables controlled filtration through a standardized ab externo approach, implant-free, with no conjunctival dissection, and minimal tissue disruption. Its proprietary notch design excises a precise segment of scleral tissue to establish a full-thickness, patent channel from the anterior chamber to the subconjunctival space. The result is a posterior, low-lying, diffuse bleb. In effect, AquaLumen replicates the core mechanism of trabeculectomy but in a minimally invasive manner, bypassing scleral flaps and conjunctival dissection to reduce unpredictable healing responses and complications that compromise long-term efficacy.
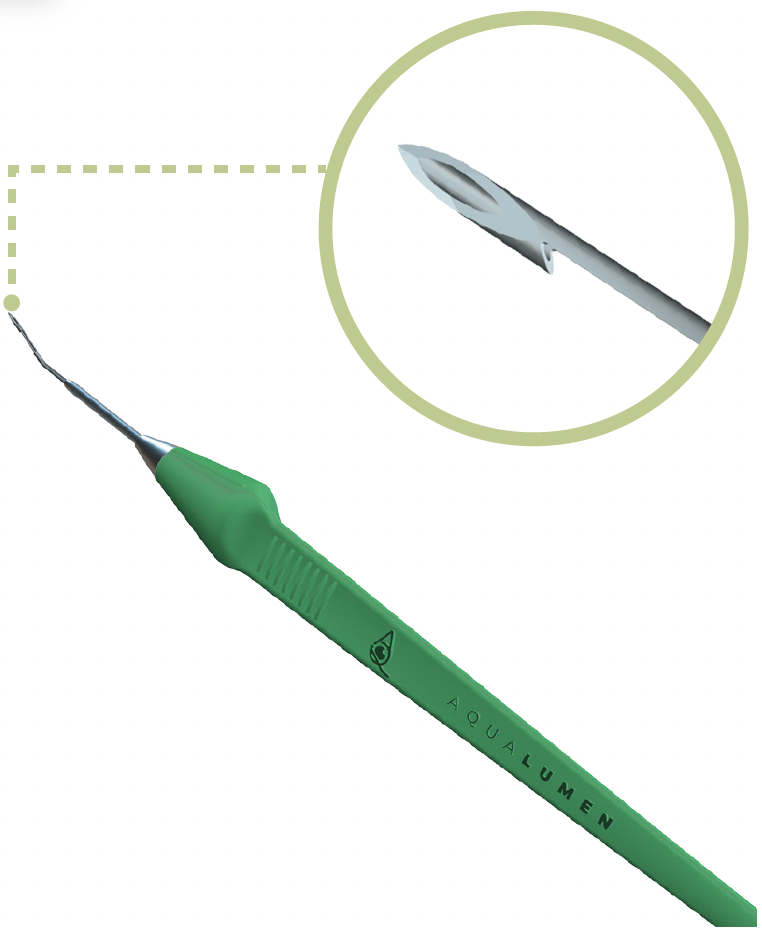
Figure. The unique cutting notch of the AquaLumen™ excises a uniform channel through the sclera.
INTEGRATION AND TRAINING
Feedback from early adopters has been resoundingly positive. Surgeons report seamless integration into standalone and combined cataract procedures, highlighting efficiency and control. Postoperatively, they describe eyes treated with AquaLumen as “quiet,” with minimal inflammation and low follow-up complexity. Because the technique mirrors familiar elements of traditional filtration surgery, the learning curve is streamlined. Our support includes peer-to-peer guidance, training videos, wet labs, and OR observation to build clinician confidence and consistency.
CLINICAL EFFICACY AND SAFETY
In a real-world retrospective case series with follow-up extending to 24 months, AquaLumen demonstrated meaningful and durable pressure control, with up to 51.2% IOP reduction at 12 months and pressures maintained in the low teens in some eyes at 24 months. Medication burden fell from an average of 3.2 preoperatively to 0.6 at 12 months, with most patients medication-free in the early postoperative period. Importantly, these outcomes were achieved in advanced glaucoma patients, many on maximal medical therapy and with prior failed procedures, underscoring AquaLumen’s potential to deliver filtration-level efficacy through a minimally invasive, implant-free approach.1 By preserving conjunctival integrity, eliminating sutures, and minimizing inflammation, the procedure is designed to support safety and a streamlined surgical experience. Consistent outcomes across multiple surgeons further reinforce confidence in the reproducibility of the technique.
PIPELINE AND REGULATORY OUTLOOK
AquaLumen is registered with the FDA as a Class I medical device and is commercially available in the U.S., with more than 200 cases already completed. A prospective study is underway at the MUSC Storm Eye Institute under the direction of Professor Jella An, MD, to evaluate 12-month outcomes in eyes with primary open-angle glaucoma. In parallel, we are in discussions with strategic and international partners to explore broader expansion opportunities.
WHERE WE’RE HEADED
AquaLumen fills a critical gap as a less invasive transcleral option for patients who are dependent on or intolerant of drops, have failed prior laser or angle-based procedures, or wish to avoid a trabeculectomy or tubes. To surgeons who hesitate to do filtration procedures: you no longer have to choose between efficacy and safety. AquaLumen introduces a reproducible, controlled alternative that preserves the conjunctiva while delivering outcomes suitable for moderate-to-severe glaucoma.
1. Data on file, PLU Ophthalmic, 2024.
For more information: https://aqualumen.net/"
For inquiries, contact Rachel Anderson at randerson@pluophthalmic.com
