
Sjögren syndrome (SS) is a chronic inflammatory disorder that may occur alone (primary) or in association with another autoimmune disease (secondary). The precise etiology is unknown, although a genetic predisposition has been suggested to increase relative risk in some cases.1 SS affects about 4 million Americans, and it is estimated that 1 in 10 cases of clinically significant aqueous-deficient dry eye (ADDE) is associated with underlying SS.2 Serious ocular manifestations, including conjunctival and corneal inflammation, uveitis, scleritis, optic neuritis, and retinal vasculitis, can result in decreased vision or permanent vision loss.3 Although associated with more severe ocular signs, such as advanced corneal staining, patients with SS-related ADDE commonly present with symptoms that are less severe than other forms of dry eye and disproportionate to their ocular surface findings.4,5 This suggests that proinflammatory factors from the disease may consequentially reduce corneal sensation.6
Background
A 57-year-old white woman presented as a referral for dry eye disease. She reported an exacerbation of symptoms in the last few months, rendering her unable to drive. She reported her pain level to be 6/10. Her medical history was significant for pemphigus, for which she occasionally took prednisone for skin flares, and hypothyroidism, controlled with levothyroxine. Her surgical history included LASIK OU (2002) and bariatric surgery. Previous treatments for her dry eye disease included warm compresses, Lotemax QID OU for 2 weeks, omega-3 fatty acids, lid scrubs, Retaine artificial tears (OcuSoft) up to 6 times per day, and lubrication ointment, all of which provided minimal improvement.
On ocular examination, visual acuity was 20/50+2 OD and 20/25 OS. The right eye revealed 4+ corneal staining with approximately 14 corneal filaments along the superior aspect of the cornea (Figure 1). OSDI scoring was 77, indicating severe dry eye disease. Meibography showed severely truncated glands with dropout to both upper and lower eyelids. InflammaDry (Quidel) was strong positive OD, weak positive OS. I classified the patient as a Type II skin type by the Fitzpatrick grading scale. Schirmer testing with anesthetic revealed <5 mm wetting OU. The patient was diagnosed with filamentary keratitis OD, meibomian gland dysfunction OU, and keratoconjunctivitis sicca OU (pending additional Sjögren’s testing).
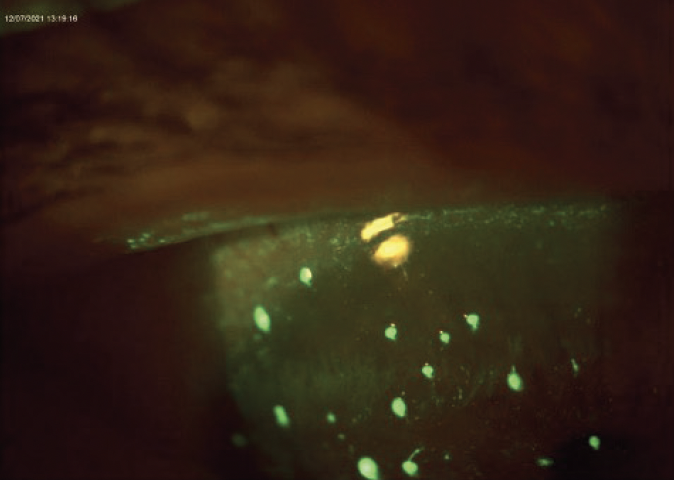
Figure 1. Anterior segment findings in the right eye revealed 4+ corneal staining with approximately 14 corneal filaments along the superior aspect of the cornea.
Treatment and Follow-Up
I treated the patient’s filamentary keratitis with a dry amniotic membrane with overlying bandage contact lens, which I removed after 6 days (Figure 2). I encouraged the patient to apply copious lubrication with Retaine MGD artificial tears. After the amniotic membrane, I started the patient on acetylcysteine TID OD and Regener-Eyes LITE Ophthalmic Solution (Regener-Eyes) TID OU. I am treating her underlying meibomian gland dysfunction by IntensePulsed Light (IPL) therapy. A laboratory panel was ordered to evaluate for SS.

Figure 2. The patient’s filamentary keratitis was treated with a dry amniotic membrane with overlying bandage contact lens, which was removed after 6 days.
The following bloodwork was ordered: Early Sjögren Syndrome Profile (PSP, CA6, SP-1) IgG/M/A for each; Sjögren Ab Anti-SS-A and Anti-SS-B; Anti-Nuclear Antibody (with Hep-2 cell pattern analysis); and Rheumatoid Factor IgM. Results showed elevated salivary protein 1 (SP-1) IgG and IgM Abs, elevated carbonic anhydrase 6 IgG and IgM Abs, and elevated parotid secretory protein IgM Abs. Sjögren Ab Anti-SS-A and Anti-SS-B, Anti-Nuclear Antibody (Hep-2), and Rheumatoid Factor IgM were all within normal limits. Antibodies SP-1, CA6, and PSP are known to occur earlier in the disease process than antibodies Ro or La, indicating early SS in this patient. These findings were communicated with the patient’s rheumatologist, dermatologist, and primary eye doctor for continued comanagement.
Since initiating treatment, the patient’s symptoms have subjectively improved, resulting in her feeling comfortable enough to return to driving.
Discussion
SS is considered a relatively common disease in the realm of autoimmune disorders, with an incidence between 3.9 and 5.3 per 100,000 population.7 Prevalence rates are variable throughout the literature and depend on the population and criteria used for case definition; no associated race or geographic predilection has been identified. SS has been described as occurring alone (primary) or secondary to other systemic autoimmune disorders.1 Primary SS is believed to disproportionately affect women compared to men, with mean age of onset in the fourth and fifth decades.7 Notably, more advanced age is associated with greater prevalence of SS.7 SS-related ADDE remains somewhat of a diagnostic challenge. Limitations exist with regards to repeatable, evidence-based, clinically available instruments and algorithms that are specific for SS-related dry eye.2 Furthermore, primary SS is indistinguishable in clinical presentation from its secondary occurrence. Regardless, prompt diagnosis and systemic treatment are of the utmost importance to avoid severe ocular and life-threatening complications.2
There are clues that should prompt the eye care specialist to investigate for SS. For example, when confronted with severe dry eye, inquire about additional symptoms related to glandular secretion, such as dry mouth or gynecological changes. Women of post-menopausal age or status, and patients with connective tissue autoimmune disease, are at higher risk. In this case, the previous diagnosis of pemphigus was a sign of a potential comorbid autoimmune disease that prompted a more extensive laboratory work-up, including testing for SS.
There are three main clinical lessons that I have learned while co-managing this patient’s case:
- Lesson One: Choose your tools wisely. I initially used a cotton tip applicator to remove the corneal filaments, but I believe this method only depressed the epithelium and resulted in recurrence. In the future, I will reach for jewelers forceps or my trusty Tweezerman tweezers to excise the filaments with cleaner margins.
- Lesson Two: It’s a matter of trust. When prescribing acetylcysteine to treat the filamentary keratitis, it took 3 weeks for the pharmacy to fill the script, ultimately delaying our intended treatment. In future practice, I’ll remember the importance of establishing a relationship with a trustworthy compounding pharmacy.
- Lesson Three: Implement what’s known and novel. It’s been 10 years since novel autoantibodies PSP, CA6, and SP-1 were first proposed as early biomarkers for SS.8 The benefit of performing laboratory testing for these markers is two-fold. First, PSP, CA6 and SP-1 are thought to occur earlier in the disease process.9 Second, novel autoantibodies can be detected in patients who traditionally test negative for anti-Ro or La, as seen in this case. In communication with rheumatology, the combined effect could result in a more prompt diagnosis of SS and thereby treatment of the underlying systemic disease.
1. Carsons SE, Patel BC. Sjogren syndrome. [Updated 2021 Nov 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431049/. Accessed May 19, 2022.
2. Akpek EK, Bunya VY, Saldanha IJ. Sjögren’s Syndrome: More than just dry eye. Cornea. 2019;38(5):658-661.
3. Akpek EK, Mathews P, Hahn S, et al. Ocular and systemic morbidity in a longitudinal cohort of Sjogren’s syndrome. Ophthalmology. 2015;122:56–61.
4. Foulks GN, Forstot SL, Donshik PC, et al. Clinical guidelines for management of dry eye associated with Sjögren disease. Ocul Surf. 2015;13:118-132.
5. Pflugfelder SC, Jones D, Ji Z, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201-211.
6. Adatia FA, Michaeli-Cohen A, Naor J, et al. Correlation between corneal sensitivity, subjective dry eye symptoms and corneal staining in Sjögren’s syndrome. Can J Ophthalmol. 2004;39:767-771.
7. Patel R, Shahane A. The epidemiology of Sjögren’s syndrome. Clin Epidemiol. 2014;6:247-255.
8. Shen L, Suresh L, Lindemann M, et al. Novel autoantibodies in Sjogren’s syndrome. Clin Immunol. 2012;145(3):251-5.
9. De Langhe E, Bossuyt X, Shen L, et al. Evaluation of autoantibodies in patients with primary and secondary Sjogren’s Syndrome. Open Rheumatol J. 2017;11:10-15.


