

Perioperative Ocular Parameters Associated With Persistent Dry Eye Symptoms After Cataract Surgery
Choi YJ, Park SY, Jun I, et al1
ABSTRACT SUMMARY
Choi and colleagues evaluated perioperative dry eye disease (DED) and meibomian gland dysfunction (MGD) parameters and their association with persistent symptoms after phacoemulsification with IOL implantation. The study included 116 eyes of 116 patients who underwent uncomplicated cataract surgery and who had no history of ocular comorbidity or use of topical eye drops other than artificial tears. Parameters including lipid layer thickness, meibomian gland (MG) dropout, tear breakup time (TBUT), Oxford staining score, lid margin abnormality, meibum quality, meibum expressability, orifice obstruction, MGD stage, Ocular Surface Disease Index (OSDI) score, and Schirmer test result were evaluated and recorded at baseline and at 1 and 3 months postoperative.
Statistically significant risk factors for persistent postoperative DED symptoms were high baseline OSDI score, low TBUT and MG orifice obstruction scores 1 month after surgery, and increased MG dropout. Choi and colleagues concluded that these parameters at baseline and at postoperative month 1 were important in predicting DED symptoms that would persist after cataract surgery.
STUDY IN BRIEF
• Researchers evaluated the association of perioperative dry eye disease (DED) and meibomian gland dysfunction (MGD) parameters with persistent DED symptoms after phacoemulsification. The following appeared to be risk factors for persistent postoperative DED symptoms: a high preoperative Ocular Surface Disease Index score, low tear breakup time and meibomian gland orifice obstruction scores, and increased meibomian gland dropout at 1 month after surgery.
WHY IT MATTERS
Investigators identified DED parameters and, in particular, MGD parameters as risk factors for persistent DED symptoms after cataract surgery. These findings underscore the importance of diagnosing and treating DED and MGD before surgery and of following up with further treatment postoperatively as needed. Effective therapy should optimize patient comfort, visual acuity, and satisfaction after cataract surgery.
DISCUSSION
After cataract surgery, an excellent postoperative visual outcome can be overshadowed by DED symptoms, including ocular irritation and transiently blurred vision. These problems can be attributed to alterations of the ocular surface during phacoemulsification.2 Although most of these alterations are thought to be transient, Choi et al showed that persistent symptoms are significantly associated with low TBUT and MG orifice obstruction scores3 and with increased MG dropout at 1 month after surgery. To identify patients at risk of persistent DED symptoms preoperatively (and thereby allow earlier treatment), the investigators performed regression analysis on baseline parameters and found that female sex, younger age, a high score of meibum expressability,4 a high OSDI score, and increased MG dropout were highly associated with persistent DED symptoms after phacoemulsification. These findings suggest that patients with these risk factors should be identified preoperatively and monitored closely to allow initiation of timely and appropriate treatment. MGD parameters particularly appear to play a prominent part in predicting persistent postoperative symptoms.
Prevalence of Meibomian Gland Dysfunction at the Time of Cataract Surgery
Cochener B, Cassan A, Omiel L5
ABSTRACT SUMMARY
Cochener and colleagues investigated the incidence of MGD and evaluated subjective DED symptoms in cataract surgery candidates. This prospective case series included 342 eyes of 180 patients, all of whom completed the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire. Additionally, their lipid layer thickness, partial blink rate, and gland structure were measured using the LipiView II Ocular Surface Interferometer and LipiScan Dynamic Meibomian Imager (both from Johnson & Johnson Vision). Slit-lamp examination was performed to evaluate TBUT, and investigators used the Meibomian Gland Evaluator (Johnson & Johnson Vision) to determine the quality and quantity of MG secretions and presence of MGD.
Statistically significantly higher mean lipid layer thickness (77.5 ±19.48 nm) was found to correlate with a SPEED score of less than 8 (mild DED symptoms, P < .05). In contrast, a lower mean lipid layer thickness (58.5 ±19.58 nm) was found in patients with SPEED scores greater than 8 (moderate to severe DED symptoms). Additionally, 52% of patients were found to have MGD, 50% of MGD patients were asymptomatic, and 56% of all patients had MG atrophy. MG function correlated significantly with age, symptoms, lipid layer thickness, and MG atrophy (P < .05).
STUDY IN BRIEF
• Investigators prospectively evaluated the incidence of meibomian gland dysfunction (MGD) and subjective dry eye symptoms in patients presenting for cataract surgery. Researchers found a 52% incidence of MGD in this patient population, and half of those with MGD were asymptomatic. Meanwhile, 56% of all patients showed meibomian gland atrophy, suggesting that preoperative testing of gland structure and function for obstructive MGD is needed in all patients.
WHY IT MATTERS
The link between obstructive MGD and symptoms of dry eye disease after phacoemulsification is well established. Through the preoperative identification and management of patients with MGD, including those who are asymptomatic, ophthalmologists can improve patients’ postoperative visual outcomes, comfort, and satisfaction.
DISCUSSION
MGD is one of the most frequently diagnosed conditions in clinical practice,6,7 and it is the most common cause of DED. Research has described the link between DED and phacoemulsification and has shown that a majority of patients develop DED after cataract surgery.2 As this study demonstrated, MGD exists in the majority of patients presenting for cataract surgery, and they are often asymptomatic. These findings led Cochener and colleagues to recommend a proactive approach to diagnosing obstructive MGD, including performing gland expression to evaluate MG function and using imaging to assess MG structure.
MG expression and imaging will not capture all cases of MG obstruction, however,8 because many glands have fixed, unyielding obstruction proximally or deep to at least one acinus—thought to represent periductal fibroses9-11—that remains in communication with the orifice. Called complete proximal obstruction, this type of blockage is characterized by intact expressible meibum from the gland (Figure 1).
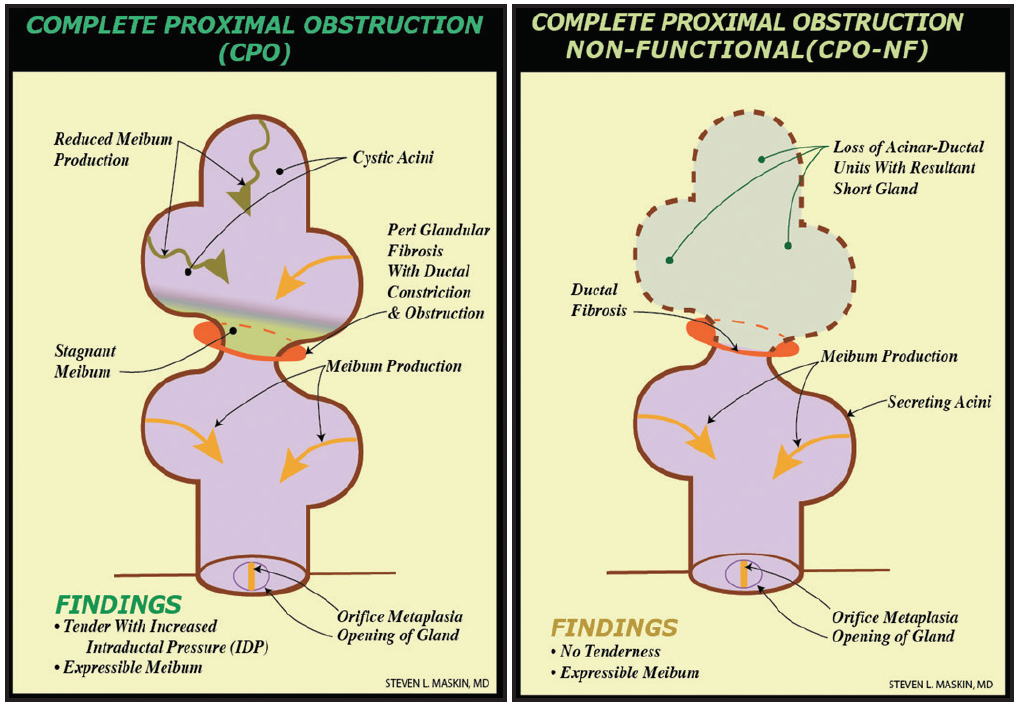
Figure 1. Proximal, fixed, unyielding MG obstruction from periductal fibroses, described as complete proximal obstruction, with resultant truncated short gland secondary to gland atrophy proximal to obstruction. (Adapted from Maskin and Testa.8)
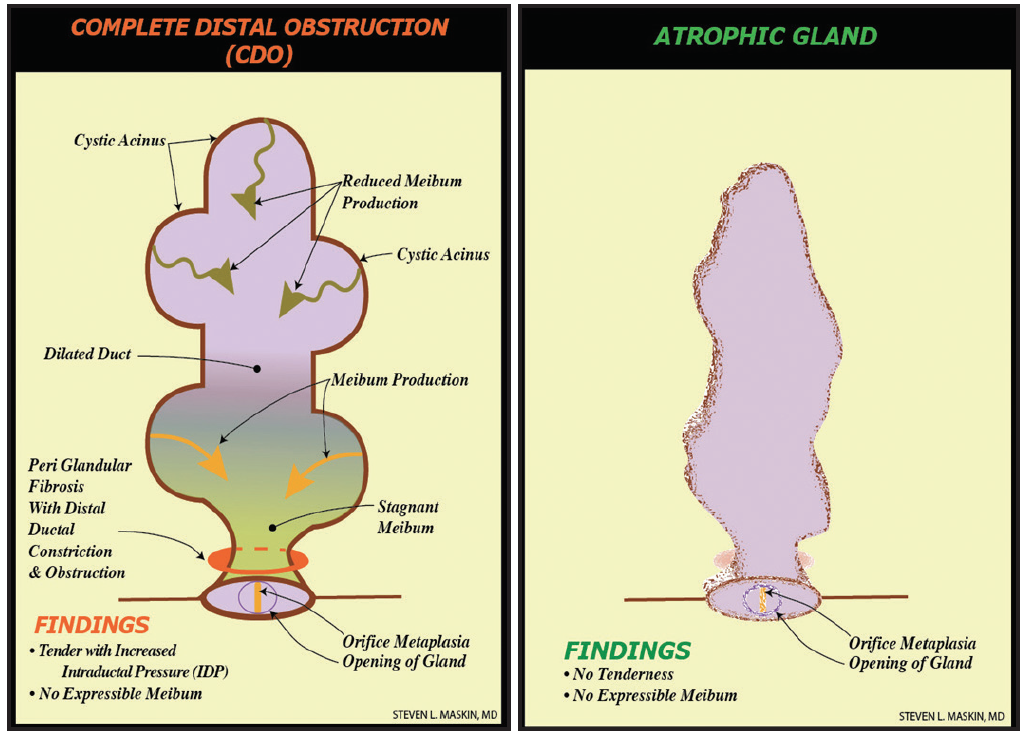
Figure 2. Distal, fixed, unyielding MG obstruction from periductal fibroses, described as complete distal obstruction, with resultant whole-gland atrophy secondary to gland atrophy proximal to obstruction. (Adapted from Maskin and Testa.8)
The only way to provide unequivocal positive physical proof of a patent meibum outflow tract without proximal or distal obstruction before cataract surgery is to probe the MG preoperatively (Figures 1 and 2). Probing also restores MG function,8 which helps prevent postoperative symptoms secondary to tear film instability, higher-order aberrations, and degraded image quality.12,13 It also helps prevent progressive obstruction from MG atrophy. Further, MG probing can promote greater comfort, better visual acuity, and improved satisfaction for patients after cataract surgery.
1. Choi YJ, Park SY, Jun I, et al. Perioperative ocular parameters associated with persistent dry eye symptoms after cataract surgery. Cornea. 2018;37(6):734-739.
2. Li XM, Hu L, Hu J, Wang W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea. 2007;26(9 suppl 1):S16-20.
3. Shimazaki J, Goto E, Ono M, et al. Meibomian gland dysfunction in patients with Sjögren syndrome. Ophthalmology. 1998;105(8):1485-1488.
4. Pflugfelder SC, Tseng SC, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17(1):38-56.
5. Cochener B, Cassan A, Omiel L. Prevalence of meibomian gland dysfunction at the time of cataract surgery. J Cataract Refract Surg. 2018;44(2):144-148.
6. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):75-92.
7. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922-1929.
8. Maskin SL, Testa WR. Growth of meibomian gland tissue after intraductal meibomian gland probing in patients with obstructive meibomian gland dysfunction. Br J Ophthalmol. 2018;102(1):59-68.
9. Maskin SL. Meibomian gland probing findings suggest fibrotic obstruction is a major cause of obstructive meibomian gland dysfunction (O-MGD). Invest Ophthalmol Vis Sci. 2012;53(14):605.
10. Cher I. Meibomian marginal dimples: clinical indicants of reactive pathogenic processes. In: Lass J, ed. Advances in Corneal Research: Selected Transections of the World Congress on the Cornea. IV. New York: Plenum Press; 1997:27-35.
11. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1(3):107-126.
12. Tutt R, Bradley A, Begley C, Thibos LN. Optical and visual impact of tear break-up in human eyes. Invest Ophthalmol Vis Sci. 2000;41(13):4117-4123.
13. Montés-Micó R. Role of the tear film in the optical quality of the human eye. J Cataract Refract Surg. 2007;33(9):1631-1635.




