White and brunescent cataracts can pose significant technical challenges in cataract surgery. The most challenging steps in dealing with these advanced, hypermature cataracts are the capsular opening—whether capsulorhexis or capsulotomy—and the nuclear disassembly.
When the femtosecond laser was introduced to cataract surgery, many surgeons saw it as a great boon for dealing with hypermature white cataracts.1 The laser could be used to create an automated capsulotomy and to fragment the nucleus, making those two most challenging steps less daunting.
Laser capsulotomy creation was especially promising because it would avoid placing stress on the capsule and zonules, decreasing the risks of tear-outs and the dreaded Argentinian flag sign, in which spontaneous capsular tears extend to the periphery. Laser fragmentation could also be beneficial to address leathery, liquefied, or rock-hard nuclei that lay beneath the capsule.
However, the laser has not been the panacea for these cases that many hoped for. Experience has shown that the plume of cortex that arises almost immediately can obscure the laser, resulting in capsular tags rather than a clean, free capsulotomy.2 Additionally, the change in the capsular architecture that occurs as liquified cortex exits the capsular bag can shift the capsule away from where the laser is firing, also leading to tags. Studies have therefore shown little difference in results between manual rhexis and femtosecond-assisted capsulotomy.2
Further, cost and access to a laser are limiting factors in the acceptance of this approach to hypermature cataracts. Therefore, although many surgeons express a preference for using laser cataract surgery in these potentially difficult cases, it is helpful to have an alternative approach or two in one’s bag of tricks. Multiple tactics have been described, and each surgeon has his or her own preferences. In this article, I summarize some popular approaches to manually opening the anterior capsule in eyes with white cataracts.
BEFORE YOU BEGIN
The first step in dealing with a hypermature white cataract is to recognize its presence preoperatively. In general there are two types: the dense, leathery nucleus and the milky white, liquefied cortex that can lead to the Argentinian flag sign.
At the slit lamp, the milky white nucleus can often be recognized by the anterior bulge of the lens capsule coming forward through the iris. A dense, leathery nucleus, on the other hand, may display some underlying brunescence, although those types of nuclei may also have some liquefied cortex. In looking at biometry, the lens thickness can also be an indicator of an intumescent lens.
MAKING THE CAPSULAR OPENING
There are many different ways to skin a cat—and to open a capsule. My own approach varies with context.
Use a 27-gauge needle. When I work alongside residents, I teach a method that I have found to be easy and straightforward. I instruct the resident to pierce the anterior capsule using a 27-gauge needle with the bevel down. The needle is attached to a syringe that has released suction, which the resident can then use to remove some of the liquified cortex to debulk the contents of the capsular bag. At that point, additional OVD can be inserted if needed, and the resident can proceed with manual capsulorhexis.
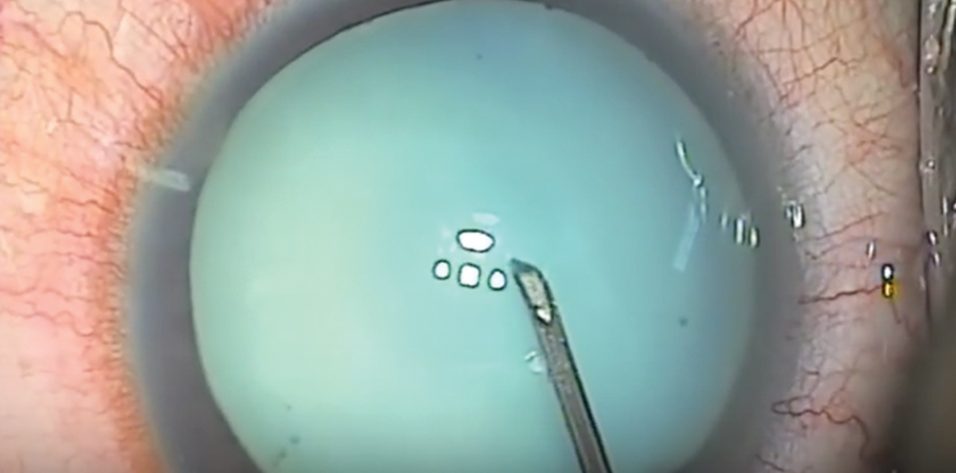
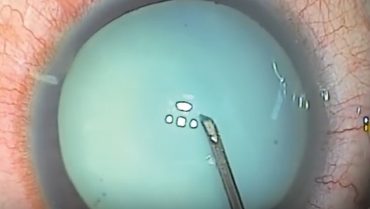
Use a cystotome needle. When I am on my own, my preferred technique is a variant of the technique I just described. I use a cystotome needle on a 3-cc syringe. I release the suction from the syringe before I enter the eye. With the cystotome, I make a small semicircular opening in the anterior capsule, so that it can’t propagate even if there is immediate egress of liquified cortex (Figure). Because I have the cystotome needle right there, I can immediately go into the nucleus and suction any liquified cortex in order to decompress the capsule and hopefully prevent the opening from tearing out. Then I can continue with the capsulorhexis from the small opening I have already made.
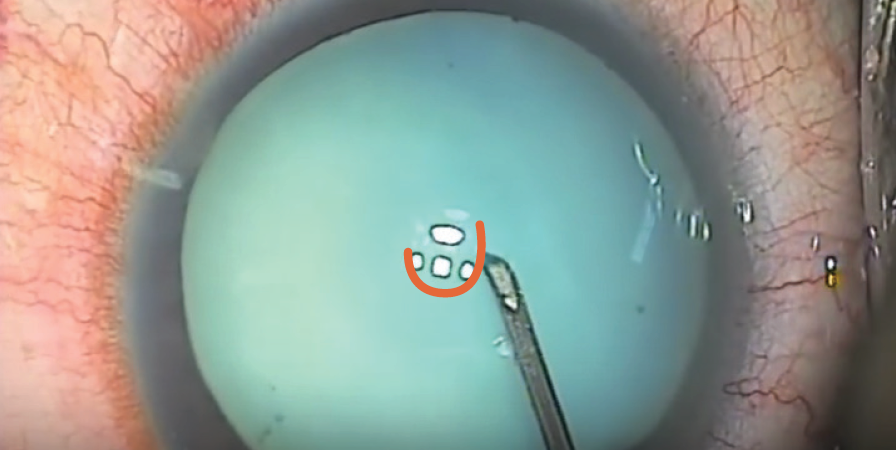
Figure. A small semicircular opening (orange) is made in the anterior capsule with a cystotome needle.
Create an OVD tamponade. Another approach in these eyes is to use a heavy OVD to tamponade the anterior capsule. The biggest benefit of this approach is that the tamponade makes the anterior chamber pressure higher than the intralenticular pressure, so it pushes the anterior capsule down and prevents the tear from going out to the periphery. In this approach, it can be advantageous to work through a paracentesis rather than through the main phaco wound, which could allow egress of the OVD.
Make a double rhexis. Videos on YouTube by Ramakrishna Tadanki, MD, of RK Eye Center in Chennai, India, and other international colleagues depict a double rhexis technique. They start by making a small rhexis, after which they can loosen any liquified cortex, remove it with a needle, and then make the larger rhexis needed to complete the phacoemulsification.
Try a phaco capsulotomy. As the name implies, phaco capsulotomy is a technique in which the phaco probe itself is used to simultaneously open the capsule and remove some of the intumescent nucleus.3 First, the anterior capsule is stained with trypan blue dye and the anterior chamber is filled with OVD. The phaco tip then enters the anterior chamber through a 2.75-mm wound without irrigation. With the bevel up, and with the probe in sculpt mode (position 3) with only torsional power (no ultrasound), the probe penetrates the capsule and begins aspirating any liquified cortex. Once the liquefied cortex is removed, the surgeon can remove the probe, refill the anterior chamber with OVD, and then proceed with capsulotomy or capsulorhexis.
Consider using a disposable capsulotomy device. Another recent technological innovation for performing capsulotomy is the Zepto (Mynosys Cellular Devices). This device includes a disposable handpiece and capsulotomy tip connected to a small control console. The nano-engineered capsulotomy tip consists of a nitinol ring covered by a soft silicone suction cup. To use the device, the anterior chamber is first filled with OVD. The tip, which is flexible and can fit through a 2.2-mm incision, is then inserted into the chamber with an attached rod, and the silicone cup attaches to the anterior capsule with application of suction. Electrical micropulsations through the nitinol ring then vaporize water molecules, cleaving the anterior capsule in a matter of seconds. When suction is released, the cap floats off the capsule and can be removed from the eye.
CONCLUSION
The femtosecond laser has no doubt been a great addition to the tool kit for the modern cataract surgeon. Still, it is not the answer to every problem. When the laser is not available, or when laser cataract surgery results in incomplete capsulotomy creation, it is helpful to have some manual capsular opening techniques to fall back on. I hope the brief summary provided here is helpful the next time you are faced with a challenging white cataract.
1. Conrad-Hengerer I, Hengerer FH, Joachim SC, Schultz T, Dick HB. Femtosecond laser–assisted cataract surgery in intumescent white cataracts. J Cataract Refract Surg. 2014;40(1):44-50.
2. Titiyal JS, Kaur M, Singh A, Arora T, Sharma N. Comparative evaluation of femtosecond laser-assisted cataract surgery and conventional phacoemulsification in white cataract. Clin Ophthalmol. 2016;22(10):1357-1364.
3. Teng CC. Phaco capsulotomy: a technique to prevent the Argentinean flag sign. Clin Ophthalmol. 2017;11:1937-1940.