CASE PRESENTATION
A 32-year-old man with type 1 diabetes presents with a recent history of vision loss in each eye. The patient reports no history of trauma. He states that his vision was good until approximately 1 year ago and that it declined dramatically in the past week.
The patient does not recall his last blood sugar level and says he checks it only when he “feels funny.” He does not know his hemoglobin A1c value. He says that he administers insulin himself according to how he feels.
He was hospitalized approximately 6 months ago, at which time his blood sugar level was 700 mmol/L. The patient has been hospitalized multiple times for uncontrolled diabetes. His first eye examination ever was 2 weeks ago with the referring optometrist.
On examination, his visual acuity is counting fingers OU that does not improve with manifest refraction. IOP readings are 15 mm Hg OU. A slit-lamp examination is unremarkable except for bilateral, white intumescent cataracts with a red reflex in each eye (Figures 1–3) but no detail visible on fundus examination. No neovascularization of the iris or angle is evident. The cataracts are remarkable for anterior capsules that are highly convex; they appear to be under pressure and bulge into the anterior chamber (AC) in front of the iris plane (Figure 4). The contents of the capsule appear to consist of opaque, white protein suspended in pockets of liquified nucleus.
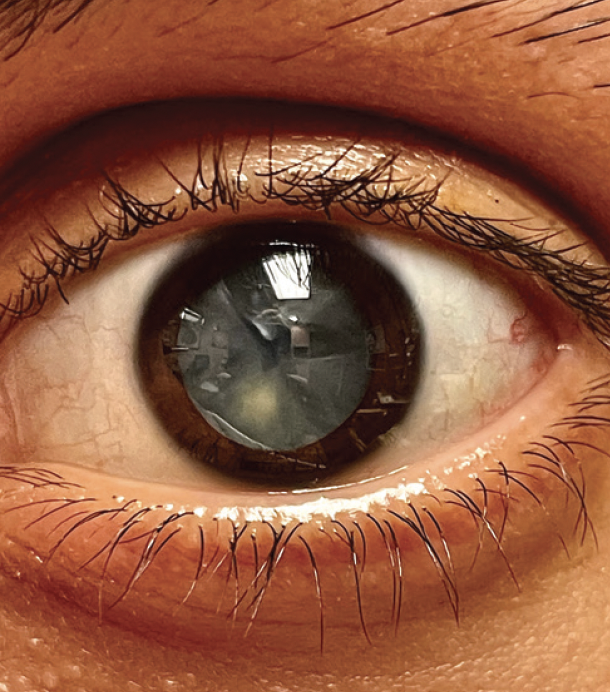
Figure 1. Fluid-filled clefts in the lens of the left eye.
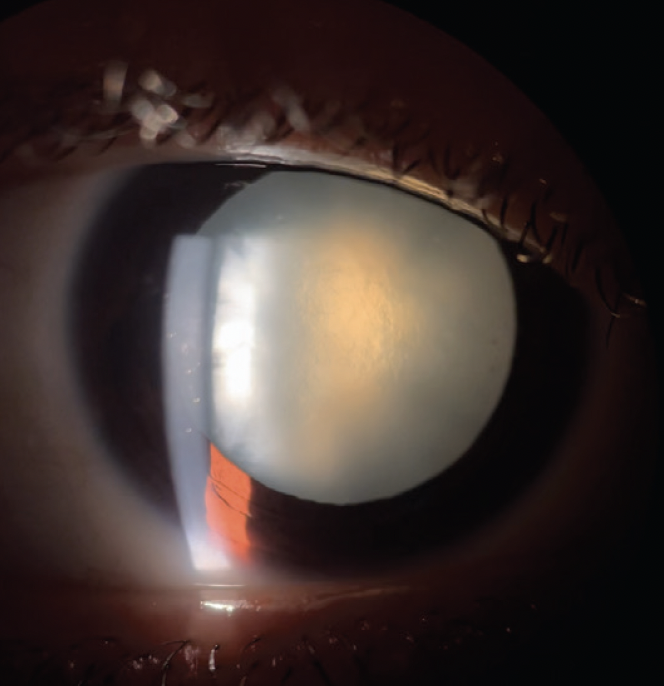
Figure 2. Slit-lamp photograph of an intumescent cataract in the right eye.
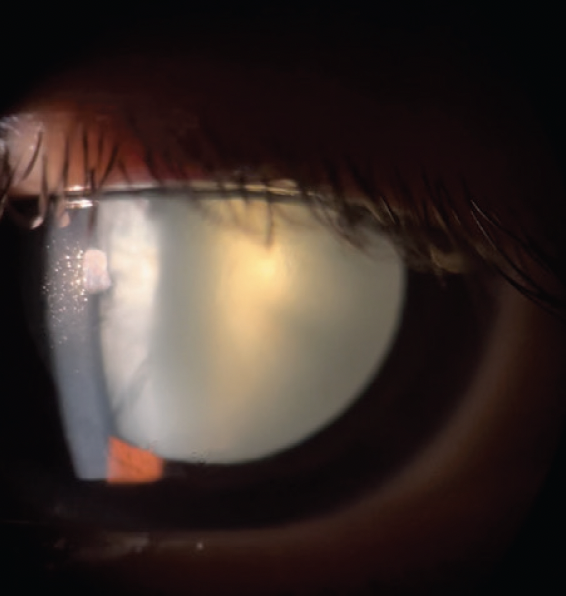
Figure 3. Slit-lamp photograph of an intumescent cataract in the left eye.
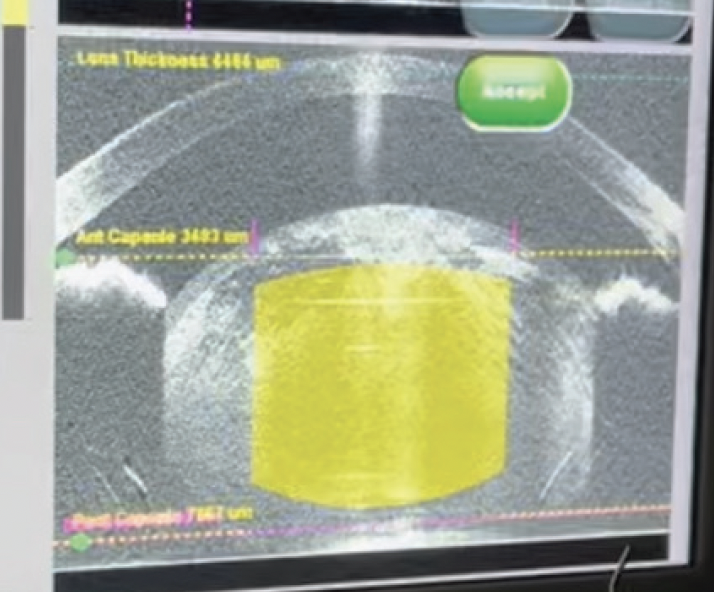
Figure 4. OCT image showing anterior bowing of the lens capsule in front of the iris plane.
A discussion with the patient covers the risks and benefits of cataract surgery, including an increased risk of rupturing the anterior capsule upon initiation of the capsulorhexis, a possible need for a vitrectomy, and the possible loss of nuclear material into the posterior segment. A decision is made to proceed with cataract extraction and IOL implantation.
How would you approach surgery? What additional testing, if any, would you perform before surgery? How would you counsel the patient?
—Case prepared by Cathleen M. McCabe, MD

ROSA BRAGA-MELE, MD, MED, FRCSC
This patient has uncontrolled diabetes and seems to have had minimal medical and ophthalmic care in the past. It therefore is important to educate him on the etiology and extent of diabetic eye disease before proceeding to surgery. His diabetic retinopathy may be extensive, including neovascularization and diabetic macular edema. This is almost impossible to diagnose accurately before surgery because of the significant cataract and an inability to visualize the retina. I would also obtain an ocular B-scan to inspect the posterior pole for vitreoretinal traction or retinal detachment. In addition to describing the risk of possible complications, I would emphasize to the patient the possibility of a poor visual outcome and the guarded visual potential due to an inability to conduct a full presurgical retinal evaluation and given the uncontrolled diabetic episodes in the past.
Surgery will be challenging. When addressing intumescent cataracts of this nature, some surgeons perform a perioperative intravenous push of mannitol to help shrink the vitreous and some of the fluid within the lens. Given the patient’s uncontrolled diabetes, however, I would advise against using intravenous mannitol because it could cause a diabetic crisis.
Sedation would be light to facilitate the patient’s cooperation during surgery and make him less likely to move suddenly. A small incision would be made, and the AC would be filled with a dispersive OVD. The anterior capsule would be painted with trypan blue dye (VisionBlue, Dutch Ophthalmic USA) underneath the OVD to optimize visibility, and the AC would be refilled with a dispersive OVD.
At this point, a 27-gauge needle on a tuberculin (1-mL) syringe would be inserted bevel down into the middle of the capsule and the liquified cortex. It is important to have some fluid in the syringe because a fluid-to-fluid interchange is easier to perform, and the withdrawal of liquefied cortex would commence immediately upon the puncturing of the anterior capsule. Verbal anesthesia would be used to discourage the patient from moving or coughing, thereby reducing the chance of a Valsalva maneuver, a sudden expansion of the anterior capsule, and a capsular tear leading to the Argentinian flag sign.
After most of the liquefied cortex had been removed, the AC would be refilled with a dispersive OVD as the needle is withdrawn. It would be important not to decompress the eye at all. Once the eye had been refilled with a dispersive OVD and the capsule flattened, a capsulorhexis that is smaller than normal (about 4 mm) would be performed. The AC would be refilled with an OVD as necessary to maintain a flat capsule, but the surgery should otherwise be relatively straightforward after this point.

HIMANI GOYAL, MD
There are many things to consider for this patient given his young age and uncontrolled diabetes. It is important to document a pupillary examination in a patient with a marked decrease in vision. An afferent pupillary defect would suggest that something in addition to the cataract is causing a potentially irreversible decrease in vision and would signal the need to curb the patient’s expectations for surgery. Given that there is no detailed view to the fundus, B-scan ultrasonography would be appropriate to view the general posterior segment anatomy. It might also be useful to confirm axial length with an A-scan because the quality of optical biometry can be affected by a white cataract.
A major concern in this situation is the high possibility of a capsular tear as soon as the capsule is punctured. If there is no red reflex, staining the capsule with trypan blue dye may be helpful. I prefer to use a dispersive OVD and to have an extra package of it open and ready to use as needed. A cystotome hook would be used to enter the eye, and the bevel would be buried in the cortex as soon as the capsule is entered. Aspiration would be initiated immediately. Decompression would continue until the capsule is no longer under tension. I have had the opportunity to do many of these cases with my residents. Sometimes, I perform decompression with a hydrodissection cannula through a paracentesis while the resident performs aspiration with a cystotome or 27-gauge needle. A femtosecond laser could be useful in this case, but this technology is not available at every facility.
If the capsule tears out and produces an Argentinian flag sign, it may be possible to create a capsular flap and circular opening in the anterior capsule with curved microscissors. Slowing down the irrigation and aspiration during cataract removal may help prevent the tear from propagating to the posterior capsule. I would have an IOL for fixation in the sulcus or sclera on hand, and I would prepare the patient for a longer or second surgery in case a vitrectomy or scleral-fixated IOL is required.
One of the most important things to do for the patient is to explain to him that he may continue to lose vision and may even lose his life from complications related to uncontrolled diabetes. Regardless of the results of cataract surgery, he must become an active partner in managing his diabetes to achieve the best outcome.

THOMAS A. OETTING, MD
The case presents the classic but tricky intumescent diabetic cataract. The obvious problem is that the lens and fluid within the lens capsule are under pressure. If the surgery is not handled carefully—and possibly even if it is—the anterior capsular tear may quickly extend to the periphery, producing the Argentinian flag sign.1 It would be reasonable to ensure that the patient’s glucose levels are well controlled before surgery. Sometimes, however, when the vision in both eyes is poor from cataracts, visual rehabilitation is essential to better glucose control because it allows the person to read the insulin syringe or glucose measuring device. In that situation, one must press on with cataract surgery on at least one eye despite poor glucose control.
I have used several strategies to try to prevent the Argentinian flag sign, including the following:
- Make a small tear initially and then use a cannula to remove lens fluid;
- Use a 27-gauge needle placed through a paracentesis to remove lens fluid and start the tear simultaneously; and
- Use the phaco needle to start the tear and remove lens fluid simultaneously.1
I am not sure which of these is best. Any of these that allows one to rock the dense nucleus to release posterior lens fluid is useful.2 After making the initial tear, injecting an OVD to flatten the anterior capsule can reduce the chance that the capsulorhexis will extend radially.
Figure 5 shows our chief resident, Caroline Wilson, MD, using a phaco needle with low vacuum settings to start a capsulorhexis. At the same time, the phaco needle bores through the capsule and releases fluid from the lens. This instrument makes a round, fairly stable hole in the capsule that can be extended later. Next, an OVD cannula is used to rock the nucleus gently and release posterior fluid. An OVD is reinjected to flatten the anterior capsule several times during the capsulorhexis as the anterior capsular tear is spiraled out to the desired diameter. The Argentinian flag sign was avoided (see video of the surgical case below). Was Dr. Wilson lucky or just that good?
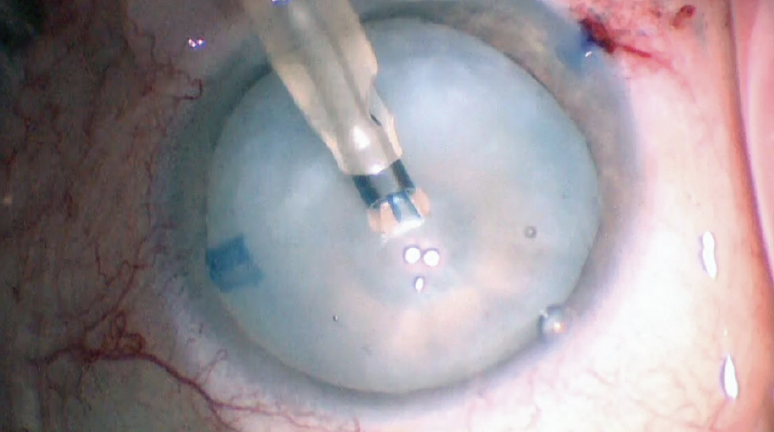
Figure 5. Caroline Wilson, MD, performs a capsulorhexis with low vacuum settings.
Courtesy of Thomas A. Oetting, MD

WHAT I DID: CATHLEEN M. MCCABE, MD
When it is impossible to view the posterior segment in an eye with a white cataract, my first step is to obtain a B-scan ultrasound to rule out intraocular pathology such as a retinal detachment or vitreous hemorrhage. In this case, the B-scan was normal in each eye. I also obtained the patient’s most recent laboratory tests, including a blood sugar of 356 mg/dL and a hemoglobin A1c value greater than 14%. The plan was to move forward with cataract surgery as soon as possible because he was unable to see well enough to administer his insulin accurately.
In this young patient with diabetes and a long history of uncontrolled blood sugar, white cortical material was suspended in pockets of liquefied nuclear material, and the capsular bag was under significant tension. Sorbitol in the capsular bag had increased the osmolarity, resulting in the diffusion of water across the capsular membrane.
Intumescent cataracts present a unique challenge during cataract surgery. There is a significant risk that the anterior capsule will rupture as soon as the bag is opened with a sharp instrument such as a cystotome. This linear anterior capsular tear has been termed the Argentinian flag sign, and it can result in posterior extension, complicating the placement of an IOL. Possible surgical approaches to decrease the risk of extension include the following:
- Flattening the dome of the capsule with a cohesive OVD and using a needle to enter the capsule and immediately withdraw fluid from the capsular bag;
- Using a phaco needle to enter the capsular bag while simultaneously performing aspiration; and
- Making a small capsulorhexis and spiraling it larger in a controlled expansion.
I have had consistent success creating a capsulotomy with a femtosecond laser in these cases. I therefore planned to execute a laser capsulotomy as quickly as possible in this case after achieving a flat dock. During the laser capsulotomy, a sudden liberation of liquefied capsule was observed, suggesting linear extension (watch a video of the surgical case below). This was confirmed in the OR, where a partial curvilinear capsulorhexis was made with Utrata forceps. The soft nuclear and cortical material was aspirated and removed with the phaco handpiece and bimanual irrigation and aspiration. A one-piece acrylic IOL was placed in the capsular bag with the haptics oriented 90º from the axis of the anterior capsular tear. Care was taken to avoid shallowing of the AC and a possible posterior extension of the tear.
One day after surgery, the patient’s UCVA was 20/30, and an examination of the posterior segment found minimal nonproliferative diabetic retinopathy.
On the second eye, I altered the plan in an attempt to avoid splitting the anterior capsule. Thirty minutes before surgery, 1 mg/kg mannitol was administered intravenously in an effort to cause deturgescence of the cataract and the vitreous. I also wanted to increase the speed of the laser capsulotomy. I considered decreasing the size of the capsulotomy to the minimum the laser was capable of (3.0 mm) but instead opted to use the Zepto (Centricity Vision), which creates a complete capsulotomy in 0.4 seconds while simultaneously applying suction. An anterior capsular extension was avoided, and the remainder of the surgery proceeded uneventfully. A one-piece hydrophobic acrylic IOL was placed in the bag.
Postoperatively, the patient’s uncorrected distance visual acuity was excellent.
1. Perrone DM. Argentinean flag sign is most common complication for intumescent cataracts. Ocular Surgery News. December 15, 2000. Accessed October 12, 2021. https://www.healio.com/news/ophthalmology/20120331/argentinean-flag-sign-is-most-common-complication-for-intumescent-cataracts
2. Vajpayee RB, Bansal A, Sharma N, Dada T, Dada VK. Phacoemulsification of white hypermature cataract. J Cataract Refract Surg. 1999;25(8):1157-1160.




