I have extensive experience with EVO ICL lenses (EVO). Talking to patients about the technology therefore comes naturally to me. Even so, the introduction of EVO has made my conversation with patients even easier because surgery is more streamlined now that a preoperative laser peripheral iridotomy is no longer required. Focus on five main points when educating patients on EVO:
- EVO is biocompatible1,2;
- EVO has a proven history of success3;
- EVO has a high rate of patient satisfaction3;
- EVO provides flexibility for the future; and
- The procedure is quick and safe.
NO. 1: EVO IS BIOCOMPATIBLE
We tell patients EVO works in harmony with their natural eye, similar to adding a lens to a camera, and explain that it is made from a collagen-containing safe and biocompatible material that also provides UV protection. We explain that EVO is soft, flexible, and invisible to them and others—making it feel like the lens isn’t even there apart from the fact that they are experiencing great vision.
NO. 2: EVO HAS A PROVEN HISTORY OF SUCCESS
Patients are educated on how the lens works. We describe that EVO functions similarly to their glasses or contact lenses to correct myopia and myopic astigmatism and that it creates sharp, clear vision by focusing light on the retina.4 We share that the technology has been used for more than 20 years and more than 2 million ICLs have been distributed worldwide.
NO. 3: EVO HAS A HIGH RATE OF PATIENT SATISFACTION
According to one survey, 99.4% of patients reported they would have the EVO procedure again.2 Patients report exceptional vision throughout the day without compromising night vision.4,5 Additionally, we share with patients that EVO is clinically proven to not cause or contribute to dry eye syndrome.6
NO. 4: EVO PROVIDES FLEXIBILITY FOR THE FUTURE
We share that EVO can be removed by a doctor, if necessary (although it is extremely rare), and does not involve removal of corneal tissue. This, we explain, ensures that they have maximum flexibility for IOL selection in the future when cataract surgery is required. On the other hand, post-LASIK patients might not qualify for premium IOLs and because making accurate IOL calculations in post–laser vision correction corneas can be challenging.
NO. 5: THE PROCEDURE IS QUICK AND MINIMALLY DISRUPTIVE
Patients like to hear that, in my hands, the EVO procedure is done in approximately 15 minutes and three easy steps (Figure 1). They are told that they will first receive eye drops to dilate and numb their eyes, and afterward, the surgeon creates a small opening at the edge of the cornea, implants EVO through the incision, and dials it into position behind the iris and in front of the natural lens to ensure proper positioning.
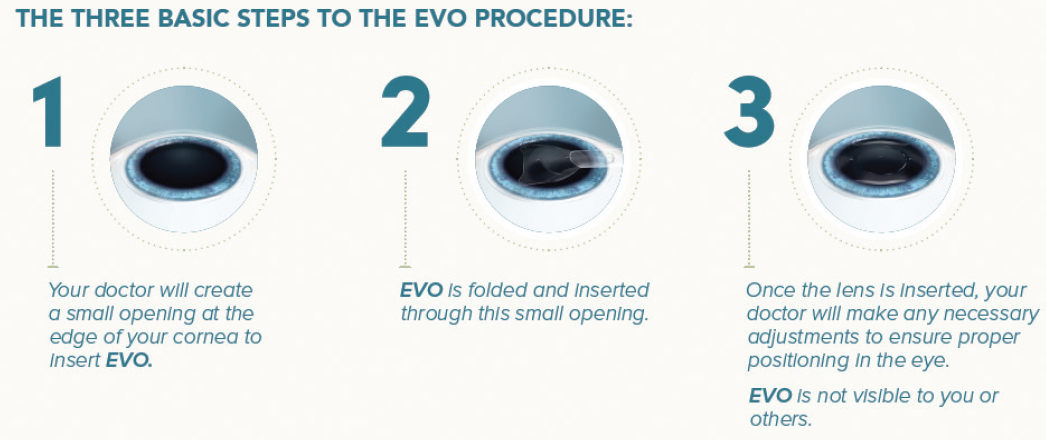
Figure 1. The three basic steps of the EVO procedure.
We talk a lot about the difference in invasiveness between EVO and laser vision correction because some patients perceive EVO as more invasive than LASIK since we actually go inside the eye during surgery. We talk to them about the amount of area that is disrupted in the cornea by making a flap as compared to EVO, where we make two very small incisions and remove no tissue. This conversation has really shifted the way patients look at the procedure.
US FDA CLINICAL TRIAL: REVIEW OF RESULTS FOR MODERATE MYOPIA
A review of the moderate myopia results from the US FDA clinical trial of the EVO confirms that the lens is accurate, predictable, and stable for the correction of moderate myopia and myopic astigmatism and that it provides high levels of uncorrected distance visual acuity (UDVA).1
A retrospective review was conducted on a subset of patients from the US FDA clinical trial. Data for 200 eyes of 114 patients (mean age, 35.1 ±5.1 years; range, 21-45 years) with a preoperative manifest refraction spherical equivalent (MRSE) between -3.00 and -6.00 D (mean, -4.61 ±0..87 D) and astigmatism up to 4.00 D who completed at least 6 months of follow-up after EVO surgery were evaluated. All procedures during the multicenter trial were conducted under US FDA Investigational Device Exemption.
At the 6-month follow-up visit, the MRSE improved to -0.085 ±0.26 D. All patients were within ±1.00 D of the intended refraction, and 91.5% were within ±0.50 D. Also at 6 months, mean UDVA and corrected distance visual acuity (CDVA) were -0.065 ±0.08 and -0.14 ±0.07 logMAR, respectively, with most eyes (98%) maintaining or gaining lines of CDVA. No eye lost more than 1 line of CDVA.
Regarding efficacy and safety, the indices were 1.03 and 1.21, respectively. There was no incidence of pupillary block, anterior subcapsular cataract, or elevated IOP due to angle narrowing or pigment dispersion. The mean endothelial cell density decreased by 2.2%, and no eye required a pre- or postoperative peripheral iridotomy or iridectomy.
1. Packer M. The EVO ICL for moderate myopia: results from the US FDA clinical trial. Clin Ophthalmol. 2022;16:3981-3991.
GOOD CANDIDATES
In the past, some surgeons reserved the ICL only for patients with high myopia and thin corneas. EVO, however, is appropriate for most patients with -3.00 D or more of myopia or myopic astigmatism. The US FDA clinical trial of EVO showed the lens provides excellent quality of vision including low and moderate myopia (see US FDA Clinical Trial: Review of Results for Moderate Myopia).7 In Hawaii, where I practice, about 70% of the population is of Asian descent. As a result, the rate of myopia is extremely high, and we don’t have to look hard to find patients who are good candidates for ICL surgery. With EVO, we have increased our pool of patients even further, allowing us to treat those who would have previously been turned away from surgery.
1. Schild G, Amon M, Abela-Formanek C, Schauersberger J, Bartl G, Kruger A. Uveal and capsular biocompatibility of a single-piece, sharp-edged hydrophilic acrylic intraocular lens with collagen (Collamer): 1-year results. J Cataract Refract Surg. 2004;30(6):1254-1258.
2. Brown DC, Ziemba SL. Collamer intraocular lens: clinical results from the US FDA core study. J Cataract Refract Surg. 2001;27(6):833-840.
3. Packer M. The Implantable Collamer Lens with a central port: review of the literature. Clin Ophthalmol. 2018;12:2427-2438.
4. Martínez-Plaza E, López-Miguel A, López-De La Rosa A, et al. Effect of the EVO+ Visian Phakic Implantable Collamer Lens on visual performance and quality of vision and life. Am J Ophthalmol. 2021;226:117-125.
5. Parkhurst GD. A prospective comparison of phakic collamer lenses and wavefront-optimized laser-assisted in situ keratomileusis for correction of myopia. Clin Ophthalmol. 2016;10:1209-1215.
6. Ganesh S, Brar S, Pawar A. Matched population comparison of visual outcomes and patient satisfaction between 3 modalities for the correction of low to moderate myopic astigmatism. Clin Ophthalmol. 2017;11:1253-1263.
7. Packer M. The EVO ICL for moderate myopia: results from the US FDA clinical trial. Clin Ophthalmol. 2022;16:3981-3991.

