


Dry Eye Disease (DED) affects millions of people worldwide. Countless treatment regimens are available globally. Many of them require daily administration, making patient adherence an issue. Tixel (Novoxel) is a novel noninvasive treatment (Figure 1) designed to reduce DED signs and symptoms. Research suggests that Tixel treatment can improve patients’ quality of life.1,2
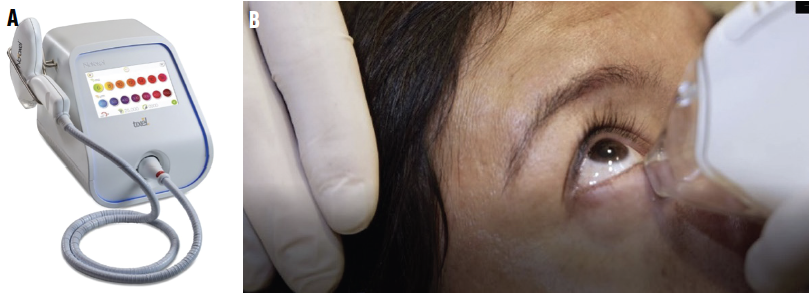
Figure 1. Tixel instrument (A). Treatment of the lower lid (B).
CLINICAL PROCEDURE
Candidates for Tixel treatment are adults with DED but no active inflammation or infection. A row consisting of six thermomechanical nonablative applications is placed directly above the margin of the upper lid. The process is repeated below the lower lid margin (Figure 2). An additional row of six applications is placed 5 mm distally to the first line. The entire procedure takes only 2 to 3 minutes to perform, and no topical cream is required after treatment (see video below to watch a demonstration).

Figure 2. Location of Tixel treatment on the lower and upper lids.
Three Tixel treatments are recommended, with an interval of 2 to 3 weeks between treatments. Lid scrubbing and expression of the meibomian glands may be added for individuals with meibomian gland dysfunction. Patients may leave the clinic immediately after undergoing Tixel treatment and resume their normal activities.
PRELIMINARY RESULTS
A major clinical trial of Tixel is underway at Vallmedic Vision & Aesthetic. A total of 61 patients with DED are receiving treatment. One of us (L.H.) is the principal investigator.
More than 75% of the patients have completed 3 months of treatment and long-term follow up. A substantial reduction in DED symptoms has been observed. Average scores on the Ocular Surface Disease Index decreased from 46.0 ±18.6 to 21.7 ±13.6. Noninvasive tear breakup time (NIBUT) increased from 9.4 ±3.0 seconds to 13.2 ±2.9 seconds (Figure 3). These results indicate that Tixel treatment significantly reduced DED symptoms and addressed the mechanisms underlying DED.1,2
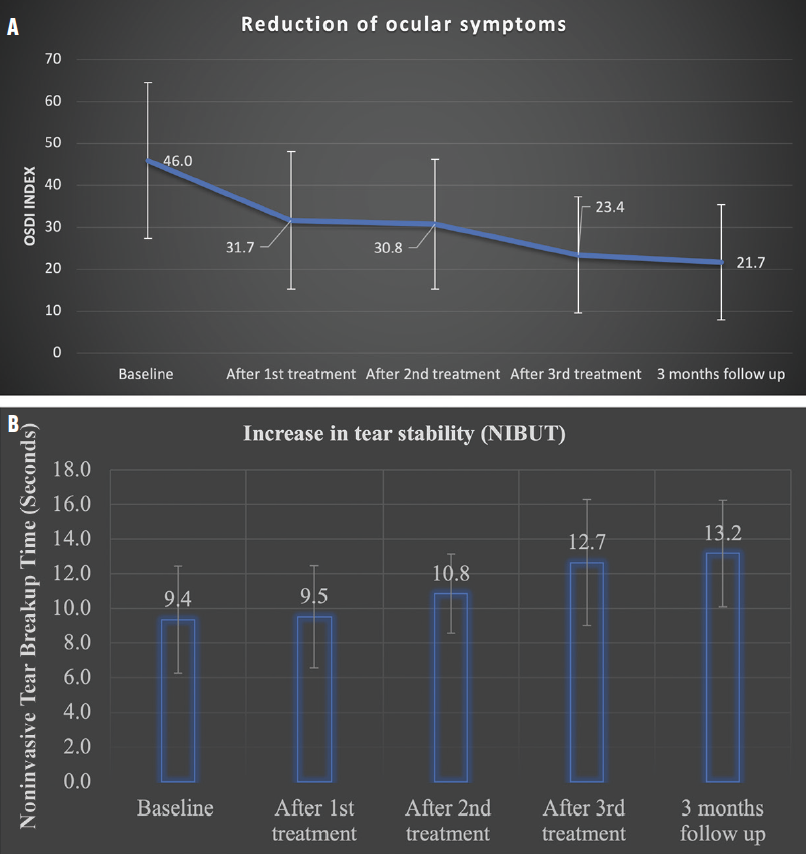
Figure 3. Reduction of DED symptoms after Tixel treatment, as indicated by changes in Ocular Surface Disease Index (OSDI) scores (A). Increase in tear film stability after treatment, as indicated by changes in noninvasive tear breakup time (B).
MECHANISM OF ACTION
Tixel delivers fractional treatment. The thermomechanical system is designed for the treatment of soft tissue of the ocular adnexa by direct conduction of heat to the surrounding tissue. The Tixel unit consists of a handpiece connected to a console by a cord. The handpiece applies a therapeutic element (ie, the tip; Figure 4), which is affixed to the distal section. The tip comprises a gold-plated copper base and a thin-walled titanium alloy cover. The handpiece is equipped with a precise motion system based on a low-inertia linear motor and a digital signal processing motion controller. The design allows the duration of skin contact to be preset.
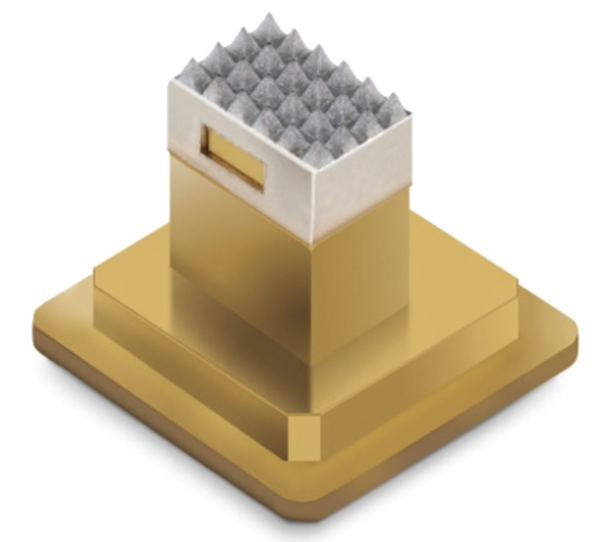
Figure 4. Device tip of the Tixel unit.
The surface area of the tip is 0.3 cm², and it features an array of 24 (6 x 4) square-based pyramids. The pyramids are 1.25 mm tall and have a flat, rectangular apex of approximately 0.01 mm2. The blunt apex of the pyramid promotes effective heat transfer and prevents mechanical puncturing of the skin. The tip’s base is warmed by a ceramic heater to a temperature of 400 ºC during treatment. Exposure of the skin to the tip lasts 6 milliseconds and creates a localized, nonablative, shallow, coagulated zone.3 Tissue coagulation takes place at temperatures of 60 to 80 ºC. This suggests that the tip’s temperature drops instantly upon contact with tissue. The thermal lesion is 200 µm deep and 325 µm wide when treatment is set for meibomian gland dysfunction.
Below 60 ºC, tissue around the ocular adnexa is heated, and the thermal energy introduced may activate nerve endings, including dermal nociceptors. This may lead to mechanical reflex blinking that results in meibum secretion. The transfer of heat may stimulate secretion from the meibomian gland via an idiopathic pathway, which requires further research.
CONCLUSION
DED frequently is not addressed sufficiently by eye care professionals, and patients can experience a long-term reduction in their quality of life as a result. DED is a chronic disorder, so periodic follow-up is required. When therapy improves patients’ symptoms, it can motivate them to see their provider regularly for maintenance.
Early results with Tixel suggest that treatment can eliminate or reduce the signs and symptoms of DED in patients who do not respond to other mainstream modalities such as intense pulsed light. The effects of Tixel treatment can last up to 9 to 12 months, suggesting that it may become an indispensable option for the treatment and management of DED.
1. Shah S, Hanneken L, Dutta D. Tixel, a completely novel way of treating dry eye. Paper presented at: The 2020 ASCRS Virtual Annual Meeting; May 16, 2020.
2. Shah S, Dutta D, Taneja M, et al. Non-ablative thermomechanical skin treatment for dry eye disease: a prospective multicentre open-label trial. Paper presented at: 39th Congress of the ESCRS; Amsterdam, Netherlands; October 8–11, 2021.
3. Elman M, Fournier N, Barnéon G, Bernstein EF, Lask G. Fractional treatment of aging skin with Tixel, a clinical and histological evaluation. J Cosmet Laser Ther. 2016;18(1):31-73.




