Case Presentation
A 37-year-old woman is referred for a LASIK evaluation. The patient is a nurse by trade. Her manifest refraction is -1.75 D of spherical error OU, and her BCVA is 20/20 OU. Her past ocular history is significant only for a mother who has a history of glaucoma. The referring physician notes that the patient has a cup-to-disc ratio of 0.45 OD and 0.55 OS. Her visual fields are normal.
The preoperative wavefront analysis (CustomVue, Johnson & Johnson Vision) is shown in Figure 1. The patient undergoes uncomplicated bilateral topography-guided LASIK. One day after surgery, her UCVA is 20/15 OU.
The referring doctor calls 3 months after surgery to report that the patient’s UCVA is 20/40 OD and 20/20-2 OS. The patient says that her vision is much worse than it was immediately after surgery. Her vision does not improve with manifest refraction. A note from the referring physician states that the IOP was 17 mm Hg OD and 18 mm Hg OS at the last examination. The doctor also reports haze in both eyes (more severe in the right eye) that is being treating with sodium chloride 5% ophthalmic solution administered four times per day and loteprednol etabonate ophthalmic gel 0.38% (Lotemax SM, Bausch + Lomb) administered once daily. No improvement has been observed. No other symptoms or subjective findings are reported by the referring doctor or patient.
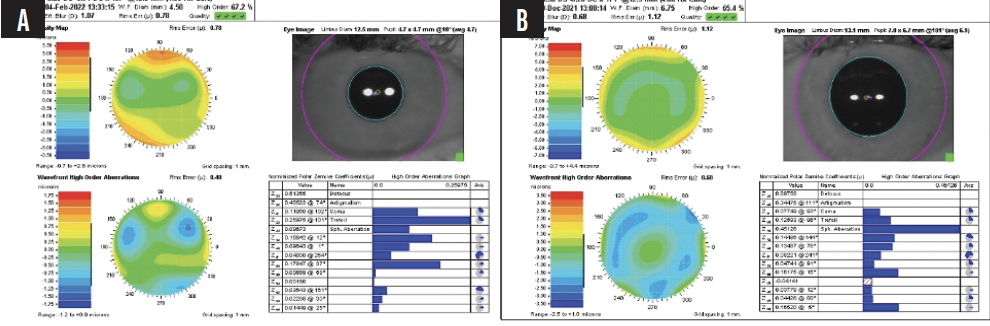
Figure 1. Preoperative wavefront analysis of the right (A) and left (B) eyes.
The patient returned to the primary surgeon (K.G.S.) with recorded off-flap pressures of 42 mm Hg OD and 32 mm Hg OS.
How would you proceed?
—Case prepared by Karl G. Stonecipher, MD; Joseph Parrish, MS; and Amy Tippin, BA

ERIC D. DONNENFELD, MD
LASIK is a safe, effective, and accurate procedure. Concern is warranted when a patient returns to the referring doctor with decreased vision that is not correctable with refraction. Pressure-induced stromal keratitis (PISK) is so rare that some surgeons may never encounter it, but the condition should be top of mind in a situation like the one described.
Topography is usually the first test performed when a patient presents with decreased vision after LASIK, and the findings are generally unremarkable in eyes with PISK. A long history of corticosteroid use is the key to making the diagnosis. Most patients with PISK are diagnosed incorrectly with diffuse lamellar keratitis (DLK). On clinical examination, an eye with PISK exhibits diffuse haze instead of the focal opacification that is usually seen with DLK, and the haze does not respond to treatment with corticosteroids. A careful evaluation may reveal fluid in the interface. A finding of low IOP is extremely helpful and, when less than 8 mm Hg, is almost pathognomonic for PISK. I have seen patients with the condition whose IOPs were as low as 2 mm Hg. An accurate IOP reading can be obtained with focal applanation in the peripheral cornea, away from the LASIK flap. I typically use a Tono-Pen (Medtronic). Occasionally, endothelial bedewing of the cornea can be visualized with fluorescein staining.
Corticosteroid therapy should be halted, and the IOP should be lowered. I usually instruct patients to administer oral acetazolamide for a few days and to begin instilling a topical glaucoma medication such as a prostaglandin analogue. Visual acuity generally recovers rapidly.

STEPHEN A. WEXLER, MD
I hear about a case of PISK from one of my referring doctors about every 6 months. They are stumped as to why a patient developed DLK months after undergoing LASIK. The patient usually has mild myopia that can be corrected to 20/20 and is always using topical steroids. The IOP is normal, as in the case presented. The reason why steroid therapy was restarted varies, but it is often to treat nonspecific red eye.
I am frequently able to diagnose PISK over the telephone by asking the doctor to recheck the IOP using a method other than Goldmann applanation tonometry or even by feeling the eye’s tactile tension under a closed lid. PISK typically resolves after steroid therapy is stopped. I also usually recommend initiating treatment with a beta blocker for about 1 week.
I have encountered cornea specialists who have no knowledge of PISK, so I make a point to discuss the condition when teaching residents or giving continuing education courses.

STEPHEN G. SLADE, MD, FACS
I believe I was a part of the first presentation on PISK when a doctor showed me a case during live surgery for a LASIK course around 1994. As noted by other panelists, the condition is rare. I know of no complications associated with it.
Surgeons who have encountered PISK know what to look for. The LASIK interface can be impressive with corneal haze and what has been described as bedewing. If the patient is administering a steroid, I stop the therapy and prescribe a topical pharmacologic treatment.
In the past, I lifted the flap, but now I typically treat PISK pharmacologically. It is important to monitor the IOP. I use a Tono-Pen, but digital palpation can be performed to confirm the diagnosis.

ARTHUR B. CUMMINGS, MB CHB, FCS(SA), MMED(OPHTH), FRCS(EDIN)
I describe PISK as the ultimate masquerader. Physicians who have not encountered the condition often treat it as DLK initially. Many of them measure the IOP using standard methods and find it to be normal. I see a patient with PISK every few years.
Steroid therapy is stopped as quickly as possible. If the patient has been administering the steroid for 7 days or less, I halt dosing abruptly. Otherwise, dosing is tapered much quicker than planned—typically under cover of a topical beta blocker. I avoid prostaglandin analogues because some inflammation may already be present. In my experience, the response to management is typically excellent, with complete resolution of symptoms and signs within 7 to 14 days of stopping the steroid therapy.

JOHN F. DOANE, MD, FACS
I rarely encounter a case of PISK. Appropriate management after diagnosis is a cessation of topical corticosteroids and initiation of aqueous suppressants if indicated by high IOP.
I consider PISK to be a good teaching topic for everyone involved in the care of patients who have a history of lamellar surgery. The interface is an anatomic entity that can be involved in pathology years after surgery.
DLK can also develop long after lamellar surgery is performed. If a patient with a history of lamellar corneal surgery develops an epithelial defect, I have a high index of suspicion for DLK. The inflammatory reaction can deliver white blood cells to the interface, and the appearance is that of DLK in the early postoperative period. If an epithelial defect develops in a patient who underwent lamellar corneal surgery—even years or decades ago—prophylactic corticosteroids should be considered. If therapy is not initiated, daily observation is warranted to ensure that DLK does not occur.

MARK LOBANOFF, MD
I see perhaps one case of PISK every year or 2. Patients typically present with persistently blurred vision about 4 to 6 weeks after LASIK. They usually had good vision for the first few days or the first week after surgery. Subsequently, their vision gradually became increasingly blurry, and manifest refraction produces little to no improvement.
The cornea may appear clear on a cursory slit-lamp examination, but a careful examination with a bright slit-lamp beam positioned at a slightly oblique angle can reveal fluid in the flap interface, usually centrally. The IOP appears to be normal if measured centrally where fluid separates the interface. Peripheral measurements, however, with a Tono-Pen or an iCare Home (Icare USA) generally find an IOP of around 30 mm Hg. OCT is my gold standard for diagnosing PISK. The scan invariably shows fluid in the interface. The steroid-induced pressure elevation in the anterior chamber drives fluid forward into the stroma, where it collects in the flap interface. Faint stromal haze due to edema is sometimes present. I have never seen inflammatory cells.
I taper the steroid drops over 3 days and start patients on an oral carbonic anhydrase inhibitor (acetazolamide) and a topical beta blocker (timolol). Fortunately, patients often recover within a week.
I prefer the term interface fluid syndrome to PISK. To me, keratitis implies the presence of inflammatory cells, which are absent in my experience.


WHAT WE DID: KARL G. STONECIPHER, MD; JOSEPH PARRISH, MS; AND AMY TIPPIN, BA
We compared preoperative images obtained with the Pentacam (Oculus Optikgeräte) with the postoperative appearance upon referral and discerned thickening of the LASIK flap (Figures 2 and 3).
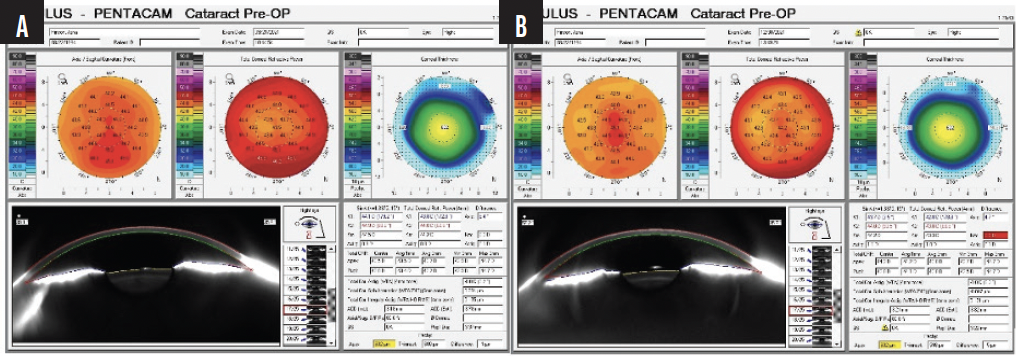
Figure 2. Pre- (A) and postoperative (B) Pentacam images of the right eye shows increased astigmatism and no change in overall corneal thickness.
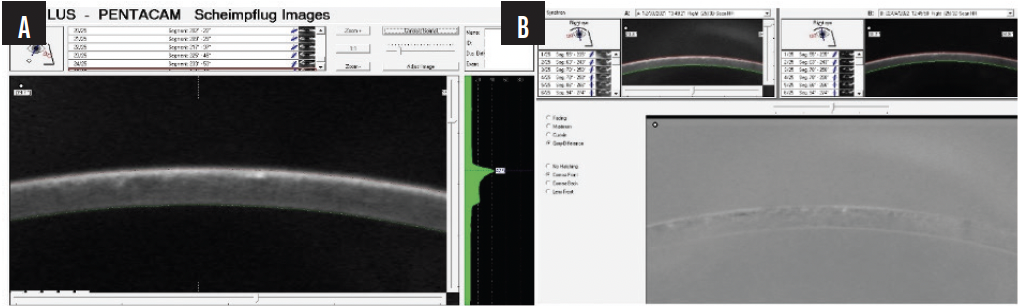
Figure 3. A Pentacam image at higher magnification shows thickening of the LASIK flap (A). The negative shows interface fluid (B).
We instructed the staff to perform off-flap IOP measurements with an iCare Home. The readings were 40 mm Hg OD and 32 mm Hg OS. The topical corticosteroids and sodium chloride 5% ophthalmic solution were stopped. Twice-daily dosing of a topical fixed combination of brimonidine tartrate and timolol maleate (Combigan, Allergan) and daily dosing of latanoprostene bunod ophthalmic solution 0.024% (Vyzulta, Bausch + Lomb) were started in both eyes.
The patient returned 1 month later. Her UCVA was 20/20 OU. Her manifest refraction was -0.25 +0.25 x 047º = 20/20 OD and plano = 20/20 OS. Figure 4 shows the topographic changes side by side. The patient reported markedly improved vision. A slit-lamp examination showed resolution of the prior findings.
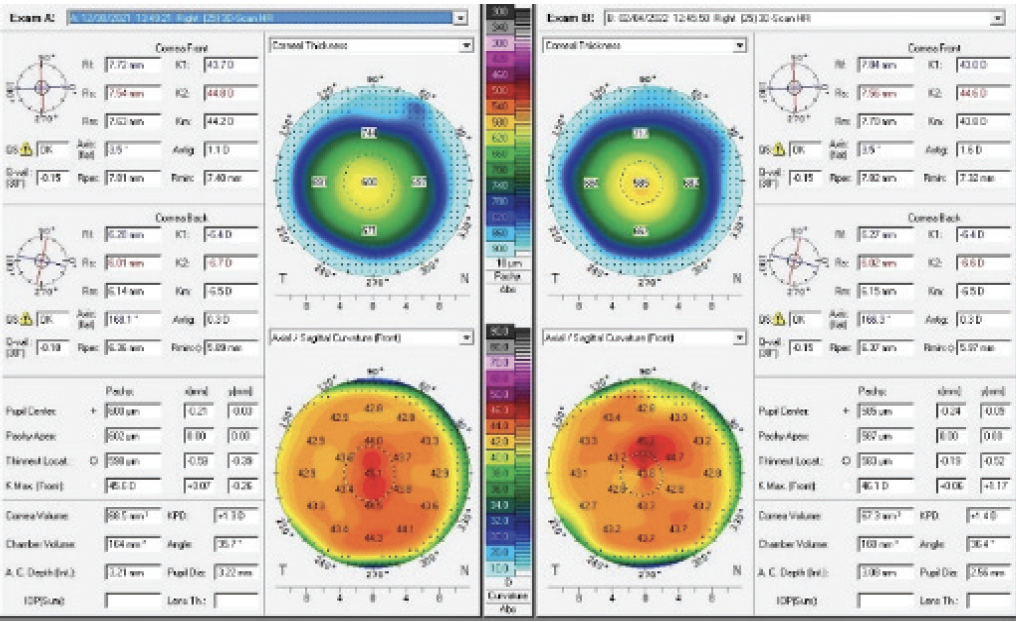
Figure 4. Resolution of the corneal thickening and a change in the overall topography.
Previously known as interface fluid edema syndrome, PISK is uncommon and often confused with DLK, as noted by the panel.1-6 When a patient with PISK is diagnosed with DLK or nonspecific haze, the first step in management is usually to increase steroid therapy, which aggravates the situation. Based on my (K.G.S.) personal statistics, PISK has occurred in 31 of my 75,147 LASIK cases and represents an incidence of 0.04% or one in 2,500.
The main factor in delayed diagnosis is that IOP measurements obtained over the flap are inaccurate, as occurred in the case presented. Tactile and off-flap IOP measurements can facilitate diagnosis. High-resolution OCT scans detect fluid in the interface in some but not all cases. Many patients, ours included, who develop PISK have a history of steroid-induced IOP elevation and/or a family history of glaucoma.
In every patient with PISK whom we’ve managed, IOP-lowering therapy has resolved the condition. Our patient will continue IOP-lowering treatment until total resolution of the symptoms and signs of PISK is achieved.
1. Portellinha W, Kuchenbuk M, Nakano K, Oliveira M. Interface fluid and diffuse corneal edema after laser in situ keratomileusis. J Refract Surg. 2001;17(2 suppl):S192-S195.
2. Lyle WA, Jin GJC, Jin Y. Interface fluid after laser in situ keratomileusis. J Refract Surg. 2003;19(4):455-459.
3. Belin MW, Hannush SB, Yau CW, Schultze RL. Elevated intraocular pressure-induced interlamellar stromal keratitis. Ophthalmology. 2002;109(10):1929-1933.
4. Galal A, Artola A, Belda J, et al. Interface corneal edema secondary to steroid-induced elevation of intraocular pressure simulating diffuse lamellar keratitis. J Refract Surg. 2006;22(5):441-447.
5. Tourtas T, Cursiefen C. "PISK-itis" or "PISK-opathy"? Cornea. 2012;31(2):107.
6. Galvis V, Tello A, Revelo ML, Valarezo P. LASIK interface complications: What is the appropriate term for PISK? J Refract Surg. 2013;29(2):81.




