
The quest continues for an accommodating IOL that can provide monofocal quality of vision from far to near without optical compromises. This article discusses five accommodating IOL designs, three of which are designed for fixation within the capsular bag and two for fixation in the sulcus plane. All of the IOLs discussed in this article are currently under investigation.
FLUIDVISION
Design. The FluidVision lens (Alcon, formerly PowerVision) is made of a flexible hydrophobic acrylic shell filled with index-matched silicone oil. The latest bench tests demonstrated that the IOL can be injected through a 3.2-mm incision with a new version of the PowerJect injection system (Alcon). A fluid-driven change in the shape of the IOL mimics the natural accommodation of the eye, creating a continuously variable monofocal lens. During accommodation, the capsular bag squeezes the fluid from the haptics into the center, inflating the lens. In the unaccommodated state, the fluid moves the other way, deflating the lens (Figure 1).
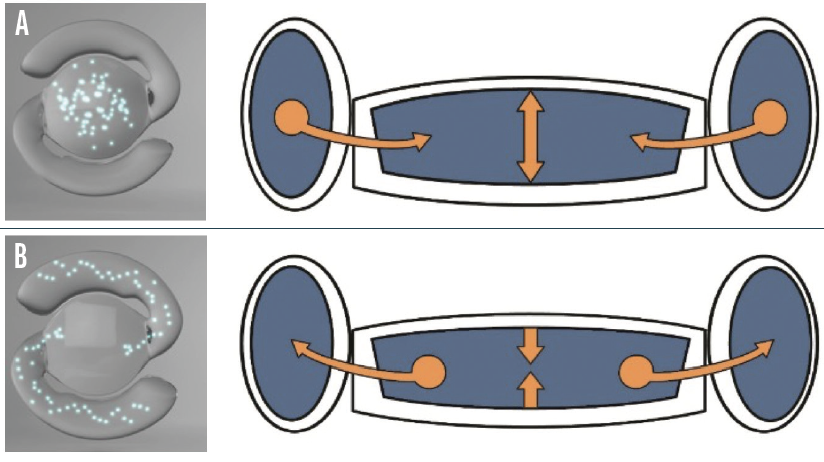
Figure 1. The FluidVision accommodating IOL in the accommodated (A) and unaccommodated (B) states.
Courtesy of PowerVision
Research. In our laboratory at the John A. Moran Eye Center, my colleagues and I performed short- and long-term studies of the IOL in a rabbit model. Our research focused on capsular biocompatibility because excessive postoperative fibrosis can impair the functioning of accommodating IOLs.1,2 It is worth noting that the rabbit is an accelerated model; 6 months in a rabbit eye corresponds to many years in a human eye in terms of posterior capsular opacification (PCO). We found significantly less opacification of the anterior and posterior capsules and overall Soemmering ring formation with the FluidVision lens compared to a standard one-piece IOL. The reason was twofold: The accommodating lens filled and significantly expanded the capsular bag, and the anterior capsule at and around the capsulorhexis edge was kept at a safe distance from the anterior IOL surface.
Single-center and multicenter clinical studies of the FluidVision IOL have been performed, and some of them are ongoing. The ORION is a prospective, multicenter study comparing the FluidVision with the AcrySof IQ IOL (Alcon). The study has shown excellent distance-corrected intermediate and near visual acuity with the FluidVision, contrast sensitivity like that obtained with the monofocal lens, and an objective amplitude of accommodation of up to 2.20 D (data on file with Alcon).
JUVENE
Design. Juvene (LensGen) is a modular accommodating silicone IOL that is composed of two components—a base lens and a fluid lens. The base lens is inserted first and fixated in the capsular bag. The fluid lens is then inserted and attached to the base lens. The dioptric power of the fluid lens changes in response to compressive forces from the capsular bag on the base lens (Figure 2A).
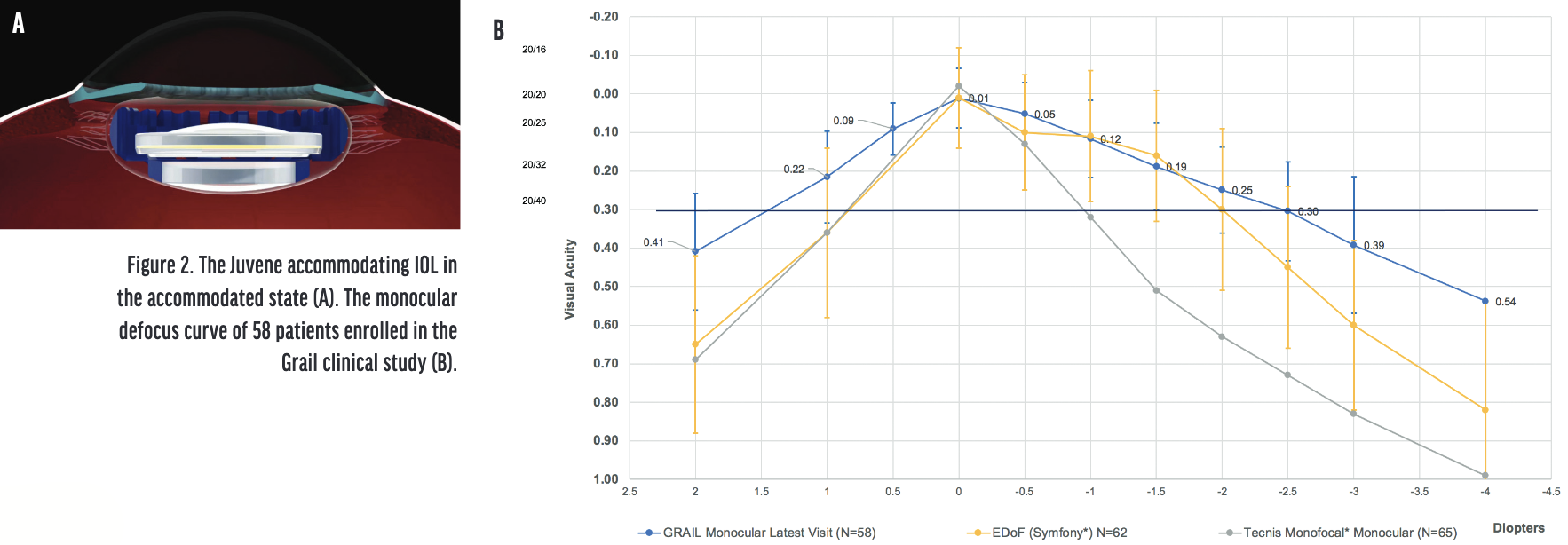
Figure 2. The Juvene accommodating IOL in the accommodated state (A). The monocular defocus curve of 58 patients enrolled in the Grail clinical study (B).
Courtesy of LensGen
Research. My colleagues and I studied the lens in our laboratory using a rabbit model. Our research focused on 6-month capsular biocompatibility and the ease of exchanging the fluid lens.3,4 PCO was scored as close to zero in the eyes that received the Juvene IOL, and all control eyes, which received a standard one-piece IOL, had the maximum PCO score at 6 months. This was confirmed clinically and pathologically. There was also significantly more Soemmering ring formation in the control eyes.
Ultrasound biomicroscopy showed that the anterior capsule remained at a distance from the anterior surface of the modular IOL system. The lack of contact kept the anterior capsule clear, whereas fibrotic changes in the anterior capsule could be seen in the control eyes. The fluid lens could be explanted or exchanged easily without significant manipulation of the base lens, capsular bag, or zonules.
Clinically, the monocular defocus curve of the Grail clinical study, with results from the last visit of 58 patients, showed that visual acuity at 40 cm was significantly better for the accommodating lens (data on file with LensGen; Figure 2B). Binocular patients saw 1 line better at intermediate and near owing to bilateral summation. After 6 months, 94% of patients had a refractive change of less than 0.50 D. Clinical studies confirmed the clarity of the capsular bag with the Juvene modular system, as suggested by the preclinical rabbit studies (data on file with LensGen).
In November 2021, LensGen received approval for an investigational device exemption from the FDA to initiate clinical studies in the United States.
ATIA VISION
Design. The Atia Vision accommodating IOL (Atia Vision) is a modular system with two optical components.5 The accommodating portion—the base—is in direct contact with the open capsular bag for efficient energy transfer from the ciliary muscle to its optic. The exchangeable front lens is a static optic that controls the final refractive power of the IOL. There is no energy transfer between the base and the front lens. Upon accommodation, fluid moves from the periphery of the base to its center, changing the shape and increasing the power of the optic (Figure 3).
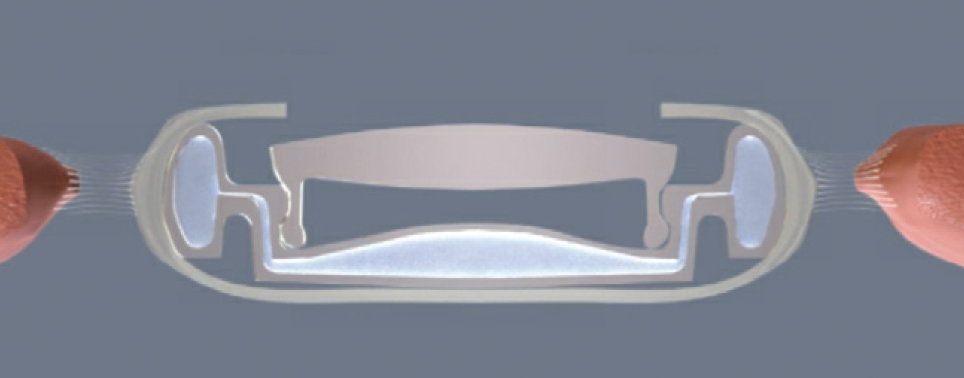
Figure 3. Schematic drawing showing the Atia Vision accommodating IOL in the accommodated state.
Courtesy of Atia Vision
Research. We are performing preclinical studies of the lens system in a rabbit model. Thus far, we have observed significant prevention of opacification of the anterior and posterior capsules with the accommodating IOL compared to standard lenses. Disengagement of the front lens from the base for exchange has been easy and atraumatic.
Ultrasound biomicroscopy showed that the anterior capsule remained at a distance from the anterior surface of the modular dual-lens system. As with the Juvene IOL, this lack of contact kept the anterior capsule clear.
Limited preliminary clinical data suggest that the Atia Vision lens has significant accommodative potential (data on file with Atia Vision). The company plans to resume clinical studies in 2022, after product optimization.
LUMINA
Design. The Lumina (AkkoLens) is a hydrophilic acrylic lens designed for positioning at the sulcus plane.6 The lens consists of two optical elements that each have an elastic omega-shaped loop with a spring function and nonelastic connections to the main body of the lens (Figure 4). When the lens is positioned at the sulcus plane, the ciliary muscle contacts the body of the lens and drives accommodation directly. The two optical elements slide in a plane perpendicular to the optical axis and produce a continuous variable-focus lens (variable-focus optics according to Alvarez7).

Figure 4. Schematic drawing of the Lumina accommodating IOL showing the overall design of the lens.
Courtesy of Jorge L. Alió, MD, PhD
In the unaccommodated state, the omega-shaped springs of the Lumina are relaxed, the optical elements overlap, the lens shows its lowest optical power, and its optical diameter is approximately 5.7 mm. When the eye accommodates, the ciliary muscle contracts and compresses the Lumina, resulting in a mutual shift of the optical elements. The optical power increases linearly with the shift, and the eye focuses at closer distances. The size of the Lumina is customized to each eye based on the measured sulcus-to-sulcus diameter.
Research. Eighty-six eyes were enrolled in a randomized clinical trial and observed for 1 year.6 The study group included 61 eyes that received a Lumina lens, and the control group included 25 eyes that received an AcrySof SA60AT monofocal IOL (Alcon). Distance UCVA and BCVA and contrast sensitivity did not differ significantly between the groups during the study period. Uncorrected near visual acuity and distance-corrected near visual acuity, however, were significantly better in the Lumina group. Defocus curves showed a statistically significant difference between groups for defocus ranging from -4.50 to -0.50 D, with significantly better visual acuities for the Lumina group.
Subjective accommodation, as determined from defocus curves, was 3.05 ±1.06, 3.87 ±1.27, and 5.59 ±1.02 D for the Lumina group and 1.46 ±0.54, 2.00 ±0.52, and 3.67 ±0.75 D for the control group at visual acuities of 0.1, 0.2, and 0.4 logMAR, respectively. The objective accommodation, measured by an open-field autorefractor, was 0.63 ±0.41, 0.69 ±0.45, 0.91 ±0.51, and 1.27 ±0.76 D for the Lumina group and 0.10 ±0.15, 0.12 ±0.15, -0.06 ±0.09, and 0.07 ±0.10 D for the control group at accommodation stimuli of 2.00, 2.50, 3.00, and 4.00 D, respectively.
The Lumina has the CE Mark. Two multicenter studies of the lens are underway in Spain and Colombia.
OPIRA
Design. Opira (ForSight Vision6) is a dynamic, shape-changing lens made of silicone that is designed for placement in the sulcus plane.5 This lens allows direct ciliary body engagement without zonular or capsular bag intermediaries. The haptics are fixated within the capsulorhexis (Figure 5A). The lens spans from the ciliary body on one side to the ciliary body on the other side. Contraction of the ciliary muscle compresses the peripheral aspect of the lens, which leads to a dynamic change in the shape of the anterior surface, thus changing the power of the IOL (Figure 5B). The static posterior lens aspect could be used to correct regular astigmatism or for postoperative refractive adjustment.
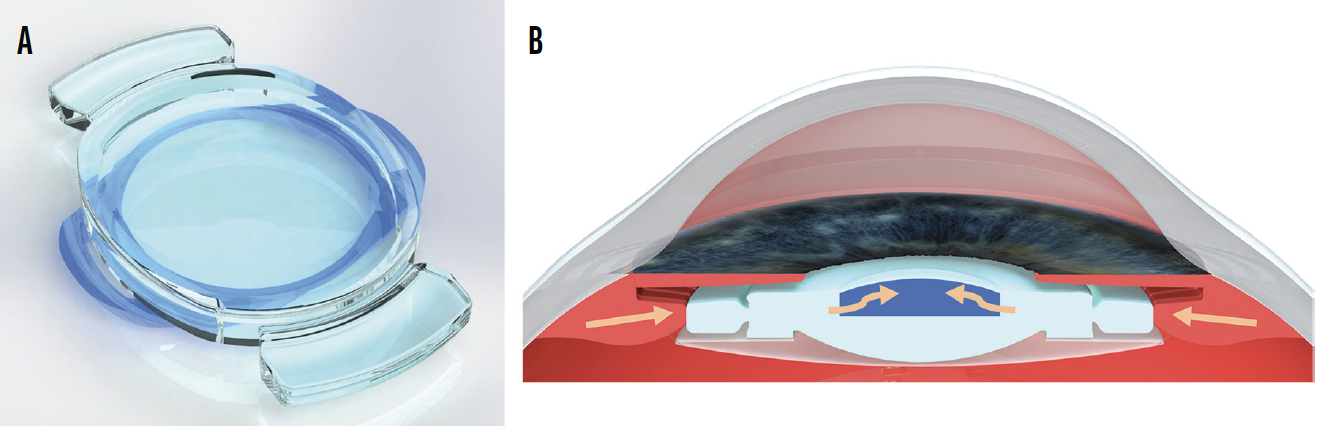
Figure 5. Overall design of the Opira accommodating IOL (A). Schematic drawing showing its site of fixation and mechanism of accommodation (B). Contraction of the ciliary muscle compresses the peripheral aspect of the lens, leading to a dynamic shape change of the anterior surface.
Courtesy of ForSight Vision6
Research. In optical bench tests, the Opira lens sustained a monofocal quality of vision across all ranges of defocus. Two years of clinical follow-up data are available for a cohort of 29 patients who received a monofocal IOL in one eye and the Opira in the contralateral eye. The eyes that received the Opira had better distance-corrected intermediate and near visual acuity than the eyes that received the monofocal lens. PCO, which occurred in many eyes, was treated with an Nd:YAG laser posterior capsulotomy without negative effects on accommodative function. Uveitis-glaucoma-hyphema syndrome has not been observed with the lens because it has remained stable in the eye and the lens’ surfaces are smooth (data on file with ForSight Vision6).
The Opira is inserted through a 3.9-mm clear corneal incision. Efforts to insert the lens through a 3-mm incision are ongoing.
CONCLUSION
The IOLs described here are nonstandard designs. There is a short learning curve in terms of implantation technique. Some progress toward smaller incisions must be made. IOLs of different sizes will have to be made available, and biometric assessment of the capsular bag or sulcus must be added to the preoperative evaluation. The long-term prevention of capsular bag fibrosis and excessive interaction with uveal tissues with related complications such as uveitis-glaucoma-hyphema syndrome are challenges with in-the-bag and sulcus-fixated designs, respectively. Because they are essentially monofocal lenses, however, accommodating IOLs remain the holy grail of presbyopia correction.
1. Floyd AM, Werner L, Liu E, et al. Capsular bag opacification with a new accommodating intraocular lens. J Cataract Refract Surg. 2013;39(9):1415-1420.
2. Kohl JC, Werner L, Ford JR, et al. Long-term uveal and capsular biocompatibility of a new accommodating intraocular lens. J Cataract Refract Surg. 2014;40(12):2113-2119.
3. Bontu S, Werner L, Kennedy S, et al. Long-term uveal and capsular biocompatibility of a new fluid-filled, modular accommodating intraocular lens. J Cataract Refract Surg. 2021;47(1):111-117.
4. Kennedy S, Werner L, Bontu S, et al. Explantation/exchange of the components of a new fluid-filled, modular, accommodating IOL. J Cataract Refract Surg. 2021;47(2):238-244.
5. Kent C. Accommodating IOLs: two more possibilities. Review of Ophthalmology. Published December 11, 2019. Accessed December 23, 2021. https://www.reviewofophthalmology.com/article/accommodating-iols-two-more-possibilities
6. Alio JL, Simonov A, Plaza-Puche AB, et al. Visual outcomes and accommodative response of the Lumina accommodative intraocular lens. Am J Ophthalmol. 2016;164:37-48.
7. Alvarez LW, inventor; Optical Res and Dev Corp, current assignee. Two-element variable-power spherical lens. US patent 3,305,294. February 21, 1967.




