CLEAR for Advanced Refractive Correction
By Jodhbir S. Mehta, BSc (Hons), MBBS, PhD, FRCOphth, FRCS(Ed), FAMS

Refractive lenticule extraction was introduced almost a decade ago. Since then, much has been learned about its clinical efficacy, safety, complications and their management, and short- to medium-term outcomes. The next evolution in refractive lenticule extraction will take many forms. One example is corneal lenticule extraction for advanced refractive correction (CLEAR), which is performed with the Femto LDV Z8 laser (Ziemer Ophthalmic Systems).
The CLEAR procedure is an optional software upgrade to the Z8 multipurpose laser, which may also be used for cataract surgery, corneal transplantation, pterygium surgery, tunnel and pocket creation for inlays, and LASIK flap creation. The CLEAR procedure received the CE Mark in April 2020 for the correction of -0.50 to -10.00 D sphere and up to -5.00 D cylinder.
ADVANTAGES
Treatment centration. One advantage of CLEAR is that it offers a wide range of centration options (Figure 1).1,2 The Femto LDV Z8 allows repositioning of the planned lenticule with the touchscreen after suction is activated. Currently, the lenticule may be centered on the pupil, the internal fixation light, or any position on the corneal surface that has been previously marked. The lenticule can also be rotated (Figure 2) to compensate for cyclotorsion when the patient is supine, which should improve treatment accuracy in patients with astigmatism.

Figure 1. Different centration options with the CLEAR procedure.
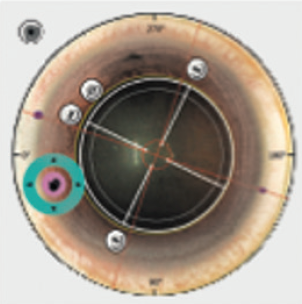
Figure 2. Compensation for cyclotorsion.
Guidance. Several incisions can be made during refractive lenticule extraction. Most surgeons either create a single 2- to 5-mm incision or two smaller incisions.
During the CLEAR procedure, two guiding tunnels (Figure 3) are created at any angle required, depending on corneal anatomy and how deep-set the eye is. These tunnels guide the surgeon to dissect directly into the anticipated plane (anterior and posterior) of the lenticule and ensures that the surgeon is in the correct plane. I expect this application to shorten the learning curve for surgeons new to refractive lenticule extraction. Those with more experience can transition to a single incision.
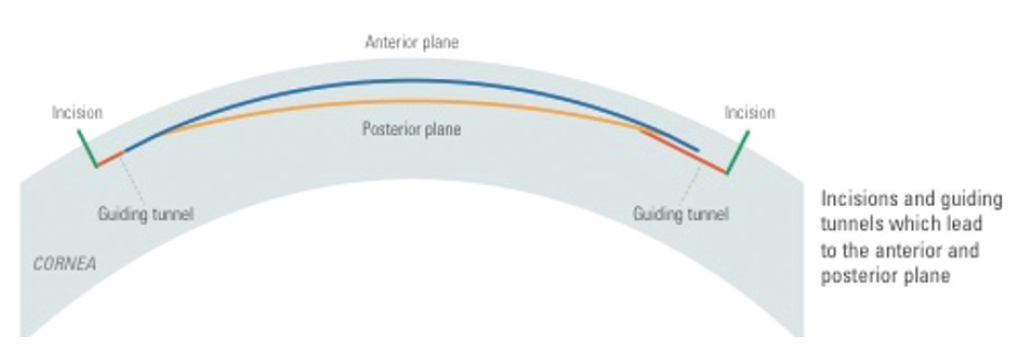
Figure 3. Incisions and guiding tunnels leading to the anterior and posterior planes.
Imaging capability. Intraoperative OCT may be performed with the Femto LDV Z8 (Figure 4). This feature is frequently used during cataract, pterygium, and transplant surgery, but it can also be useful during complex CLEAR cases such as in eyes that have a history of corneal transplantation.
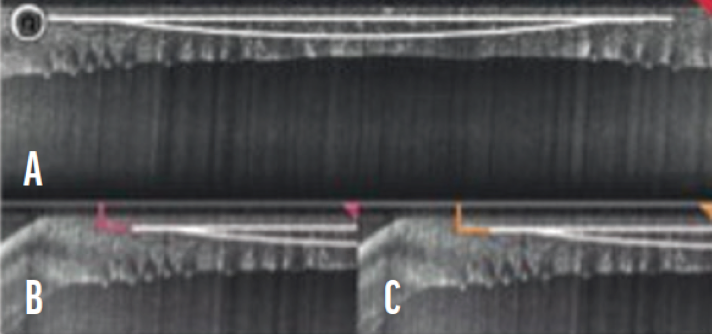
Figure 4. Intraoperative OCT scan showing planned lenticule (A), incisions (B), and guiding tunnels (C).
Figures 1–4 courtesy of Jodhbir S. Mehta, BSc (Hons), MBBS, PhD, FRCOphth, FRCS(Ed), FAMS
MY EXPERIENCE
My experience with CLEAR includes the six cases I performed before the procedure received the CE Mark. At that time, the software could create only a myopic spherical lenticule. Now, the software may be used for astigmatism correction as well. The laser head is docked in a fashion similar to when performing LASIK. Vacuum occurs after applanation, and docking should ensure that the risk of suction loss is low. After docking, the lenticule position can be adjusted and rotated, as described earlier, to ensure it is on the corneal vertex. I marked the 0º to 180º axis and the corneal vertex at the slit lamp. Lenticule creation took approximately 30 seconds.
The footprint of the laser system means that the same bed can be used under a microscope for lenticule extraction. Although there was no sidecut, I found delineating the anterior and posterior planes with a SMILE dissector (ASICO) to be straightforward. It was important to use a smooth dissector to separate residual tissue bridges, but I observed few bridges during my cases. Lenticule extraction was uncomplicated.
Postoperatively, UCVA was 20/30 or better on day 1, the interface was clear, and the incisions were well healed. An analysis of postoperative tomography showed the minimal induction of spherical aberration and vertical coma.
CONCLUSION
My initial experience with the CLEAR procedure was encouraging. Clinical results were excellent with respect to refractive outcomes and higher-order aberrations. I look forward to gaining more experience with the new software upgrade now available.
1. Kang DSY, Lee H, Reinstein DZ, et al. Comparison of the distribution of lenticule decentration following SMILE by subjective patient fixation or triple marking centration. J Refract Surg. 2018;34(7):446-452.
2. Wong JX, Wong EP, Htoon HM, Mehta JS. Intraoperative centration during small incision lenticule extraction (SMILE). Medicine. 2017;96(16):e6076.
SmartSight Offers a Twist
By Kishore Raj Pradhan, MD, and Samuel Arba Mosquera, PhD

Lenticule extraction is an established laser vision correction technique. Since its introduction 10 years ago,1 more than 3.5 million SMILE procedures have been performed with the VisuMax femtosecond laser (Carl Zeiss Meditec), and more than 600 articles on this lenticule extraction technique have been published in the peer-reviewed literature. Alternatives to SMILE are now surfacing, including Schwind eye-tech-solutions’ SmartSight procedure.
Performed with the Atos femtosecond laser (Schwind eye-tech-solutions), a platform designed to enable corneal cuts for a variety of indications,1-5 SmartSight creates a predefined lenticule in the intrastromal tissue of the cornea and makes small peripheral incisions in the topmost layer of the cornea for lenticular access. The procedure spares tissue by optimizing lenticular geometry. No lenticule sidecut is performed, and the lenticule is tapered outside the optical zone using a transition zone, much like with the Amaris (Schwind eye-tech-solutions), toward the periphery in an effort to reduce epithelial remodeling and minimize regression. Because the shape of the lenticules created during this procedure differs from those created with a VisuMax laser, even surgeons who have experience performing SMILE will face a learning curve and will need to modify their technique.
CLINICAL GUIDELINES
Current settings. Based on early experience with this technology, a track distance of 4 µm and a pulse energy of 100 nJ are currently recommended. Treatment time is 39 seconds for the SmartSight procedure, and the total energy is less than 800 mJ. The average dose is less than 0.8 J/cm2 per cut, and the average laser power is less than 85 mW.
Lenticules can be created with an energy level as low as 80 nJ per pulse (with 3-µm spacing) or with a track distance as large as 5 µm (using 125 nJ per pulse). Oversizing the cap so that it is approximately 0.7 mm wider than the transition zone provides ample room for surgical maneuvers and reduces the cap diameter (7.8–8.5 mm).
High corrections and thin corneas. To maximize the residual stromal bed, an optical zone diameter ranging from 6.0 to 6.5 mm and a thin (< 120 µm) cap are used in eyes requiring high amounts (> -7.00 D) of refractive correction (Figures 5 and 6). The same cap setting is used for eyes with thin corneas.
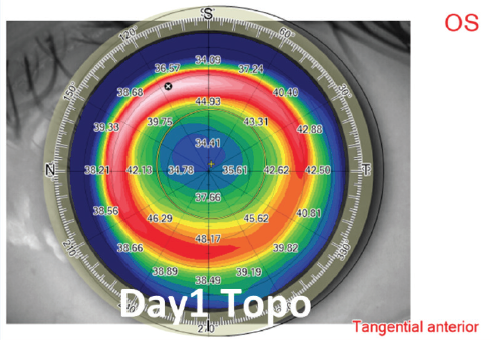
Figure 5. Example of high myopic treatment and postoperative day 1 topography.
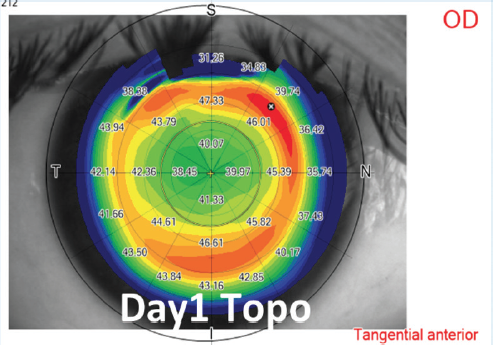
Figure 6. Example of moderate compound myopic astigmatism treatment and postoperative day 1 topography.
Low myopic corrections. For low (< -4.00 D) corrections, a large optical zone diameter (> 7.0 mm) is used to provide a better quality of vision (Figure 7).
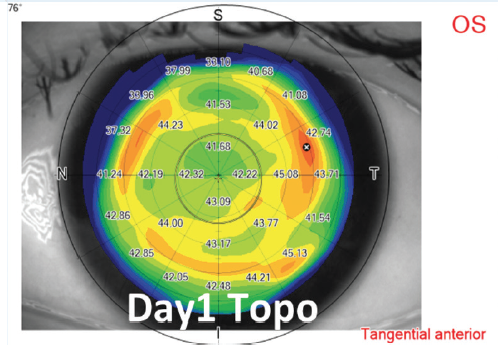
Figure 7. Example of low-to-moderate myopic treatment and postoperative day 1 topography.
Figures 5–7 courtesy of Samuel Arba Mosquera, PhD
OUTCOMES
One of us (K.R.P.) has performed the SmartSight procedure on 240 eyes.
Visual acuity. For the overall cohort, uncorrected distance visual acuity (UDVA) was 6/6 in 41%, 81%, and 90% of eyes at 1 day, 1 week, and 1 month, respectively. At 1 month, UDVA was 6/9 in 98% of eyes and 6/12 of 100% of eyes. UDVA was within 1 line of preoperative corrected distance visual acuity in 80%, 95%, and 98% of eyes at 1 day, 1 week, and 1 month, respectively.
Corrected distance visual acuity was the same or better than preoperative levels in 84%, 96%, and 94% of eyes at 1 day, 1 week, and 1 month, respectively.
Spherical equivalent. Spherical equivalent was overcorrected by 14%, 8%, and 6% at 1 day, 1 week, and 1 month, respectively. It was within ±0.50 D of target in 17%, 46%, and 62% of eyes at 1 day, 1 week, and 1 month, respectively. One month after surgery, spherical equivalent was within ±1.00 D of target in 99% of eyes.
Astigmatism. Astigmatism was undercorrected by 2% at 1 day and 1 week and by 4% at 1 month. It was within ±0.50 D of target in 97% of eyes at 1 day, 1 week, and 1 month. In all eyes, astigmatism was within ±1.00 D of target at 1 week and 1 month. The angle of error was within 15º in 99% of eyes at 1 day, 1 week, and 1 month.
COMPLICATIONS
No intraoperative loss of suction occurred, but suction was lost before treatment began for two attempted lenticules and two attempted flaps. Optical zones seemed to be larger than planned. No incisional bleeding, subconjunctival hemorrhage, tearing of the lenticule, or abrasion at the incision was observed.
An opaque bubble layer and black spots or inaccurate laser pulse placement due to eye movement were occasionally observed, but they did not interfere with dissection. Unintended initial posterior plane dissection was never complete. If the posterior plane was entered initially, the surgeon detected it, and the anterior plane was subsequently entered.
CONCLUSION
Patients who have undergone the SmartSight procedure to date have achieved good results. Based on early experience with this procedure, transition zones may be important for improving both outcomes (eg, corneal curvature gradient and higher-order aberrations) and short-term refractive stability (ie, less epithelial remodeling and undercorrection/regression).
1. Pradhan KR, Reinstein DZ, Carp GI, Archer TJ, Gobbe M, Gurung R. Femtosecond laser-assisted keyhole endokeratophakia: correction of hyperopia by implantation of an allogeneic lenticule obtained by SMILE from a myopic donor. J Refract Surg. 2013;29(11):777-778.
2. Studer HP, Pradhan KR, Reinstein DZ, et al. Biomechanical modeling of femtosecond laser keyhole endokeratophakia surgery. J Refract Surg. 2015;31(7):480-486.
3. Pradhan KR, Reinstein DZ, Vida RS, et al. Femtosecond laser-assisted small incision sutureless intrastromal lamellar keratoplasty (SILK) for corneal transplantation in keratoconus. J Refract Surg. 2019;35(10):663-671.
4. Reinstein DZ, Pradhan KR, Carp GI, et al. Small incision lenticule extraction for hyperopia: 3-month refractive and visual outcomes. J Refract Surg. 2019;35(1):24-30.
5. Pradhan KR, Reinstein DZ, Carp GI, Archer TJ, Dhungana P. Small incision lenticule extraction (SMILE) for hyperopia: 12-month refractive and visual outcomes. J Refract Surg. 2019;35(7):442-450.