
Demodex blepharitis is more prevalent than previously thought, and yet it is still dramatically underdiagnosed in real-world ophthalmology patients, according to a recent multicenter study of consecutive patients undergoing slit-lamp exams.1 However, a new treatment option, lotilaner ophthalmic solution, 0.25% (Tarsus), which is currently being reviewed for approval by the US FDA, has demonstrated impressive results in clinical trials and stands poised to significantly alter how the common lid margin disease is managed.
“Blepharitis is sort of the bane of our clinical experience,” Dr. Periman explained. “Every specialty in ophthalmology deals with it on a daily basis—or ignores it on a daily basis. And to be fair, we haven’t had super effective strategies for addressing blepharitis. But we’re starting to get things in our toolkit that make treating blepharitis more precise and effective.”
Multicenter Study Demonstrates Prevalence of Demodex Blepharitis
In a study involving seven investigators at six clinics, consecutive patients undergoing slit-lamp examinations were reviewed for Demodex blepharitis by the presence of collarettes.1 Of the 1,032 patients enrolled in the study, 57.7% were found to have Demodex blepharitis, but the investigators also found evidence that its prevalence may be higher in the general population. Perhaps unsurprisingly, 44% of patients with collarettes had not previously been diagnosed with blepharitis, suggesting that degree of symptom bother is a less accurate depiction of disease burden compared to the pathognomonic sign of collarette presence.
According to the study, the prevalence of collarettes was higher in select subgroups: patients with a known diagnosis of blepharitis (69.1%) and those with glaucoma (64.8%). Furthermore, although 64.8% were diagnosed with collarettes during an annual eye examination, 60.8% were diagnosed during an exam for glaucoma, 59.9% during a cataract evaluation, and 53.5% during a dry eye evaluation.
As part of the screening protocol for the study, patients were asked whether they were currently using tea tree oil. Of those who answered yes, 74.5% were eventually diagnosed with blepharitis; of those who answered no, 56.7% were ultimately diagnosed with blepharitis.
“The temptation is to say, well, tea tree oil doesn’t work, but maybe these patients are self-selecting due to more severe symptoms, looking for different relief and are trying different products that contain tea tree oil,” Dr. Periman said.
New Treatment Option
As is the case with many ophthalmic conditions, when viable treatments come to the clinic, specialists’ awareness of the disease tends to heighten. According to Dr. Periman, upon looking at the data from clinical trials involving lotilaner ophthalmic solution, 0.25%, it is not hard to see why eye care providers who deal with a lot of lid margin disease are very excited about how it may change clinical practice.
In a randomized, controlled, double-masked clinical trial, 54 patients with Demodex blepharitis were randomized 1:1 to topical lotilaner ophthalmic solution, 0.25%, or vehicle.2 At the topline, all outcomes were statistically significantly higher at day 42 for patients treated with topical lotilaner ophthalmic solution, 0.25%, dosed BID compared to vehicle. A closer look at the numbers, however, shows just how effective lotilaner was in eliminating collarettes, eradicating mites, clearing erythema, and achieving a statistically significantly higher rate of composite cure (73.3% vs 10.5% in vehicle; P<.001) (Figure 1).
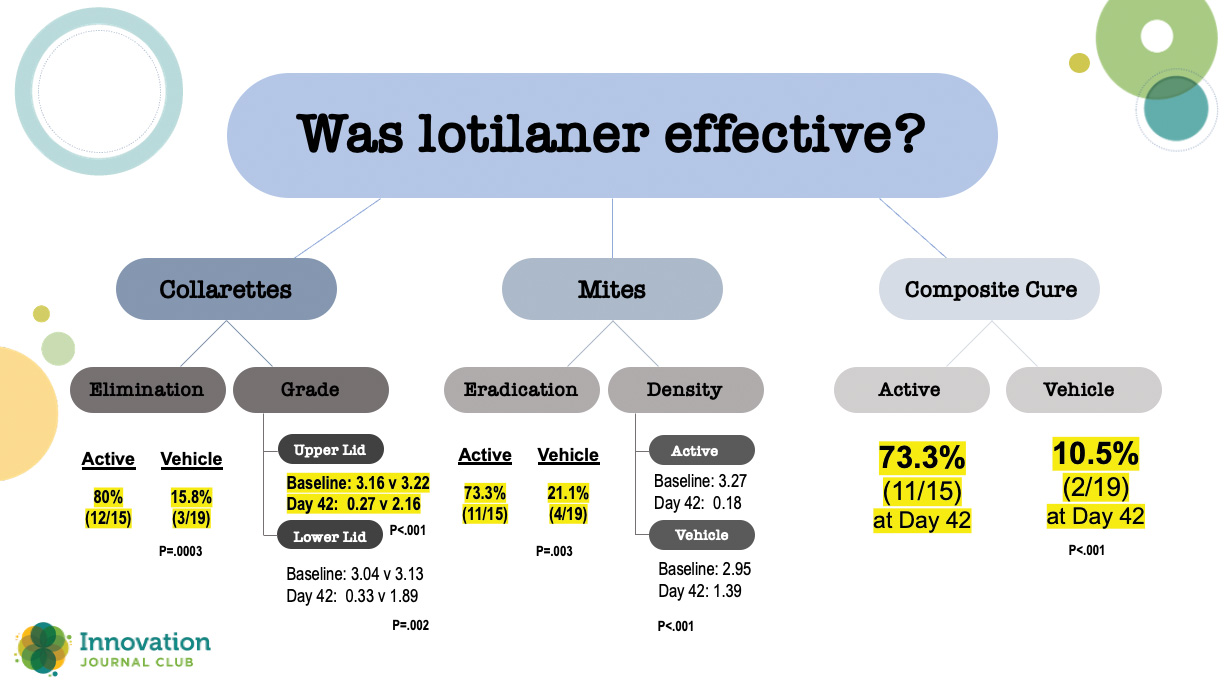
Figure 1. Results from a 54-patient randomized, controlled, double-masked clinical trial comparing lotilaner ophthalmic solution, 0.25% and vehicle.
While those results were impressive, Dr. Periman said the real proof of lotilaner’s effectiveness is in the 421-patient phase 3 Saturn-1 trial that compared lotilaner to vehicle at BID dosing. As with the other study, all outcomes were statistically significantly higher for lotilander versus vehicle (Figure 2). What was slightly unprecedented, though, was the degree of separation between the two study arms.
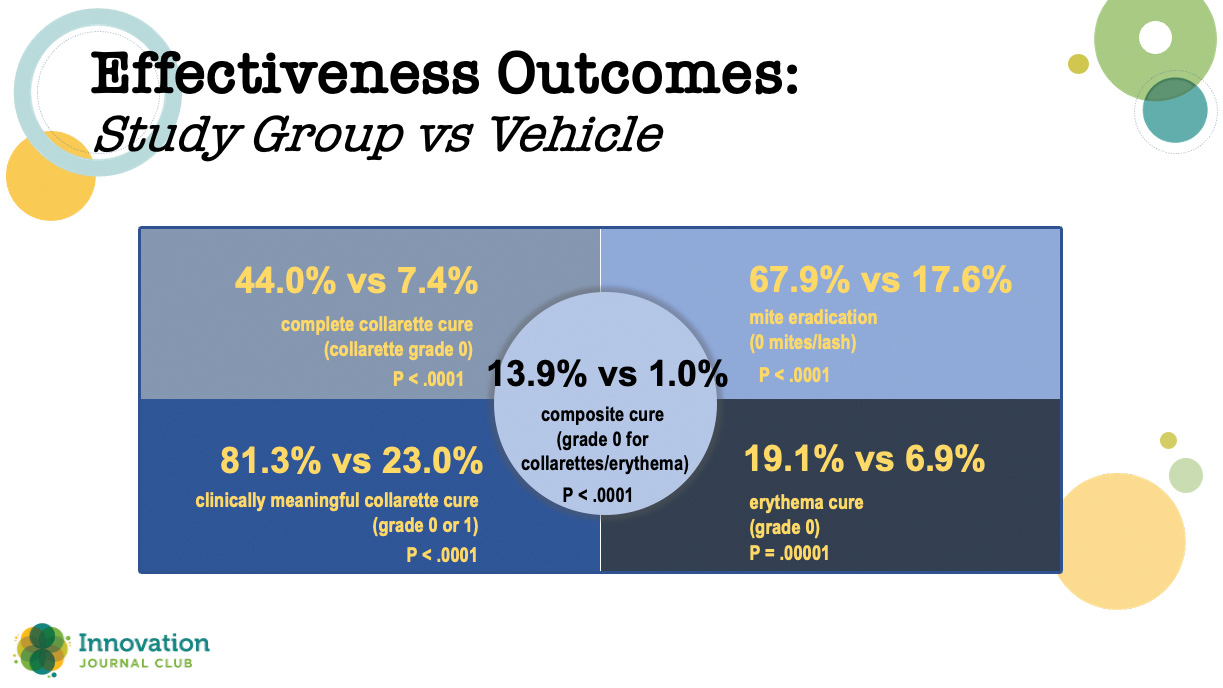
Figure 2. In the phase 3 Saturn-1 study, all outcomes were statistically significantly higher at day 43 for patients treated with topical lotilaner ophthalmic solution, 0.25% compared to vehicle.
“There are so few ocular surface disease treatment options that we have, procedure-based or drop-based, that have this kind of crazy-good data. I don’t know if I’ve seen this before in ocular surface disease,” Dr. Singh said.
Dr. Periman agreed: “When we look from a 30,000-foot view of how frustrating ocular surface disease can be for our colleagues and for patients alike, one of the reasons for that is we haven’t had targeted and specific, strategic therapeutics to address very common contributors. Turns out, Demodex blepharitis is involved in about 60% of your MGD cases. To have an eyedrop that is a 6-week course, that has an 81% clinically meaningful cure, is a very straightforward, time efficient, positively reinforcing approach for these ocular surface disease patients.”
1. Trattler W, Karpecki P, Rapoport Y, et al. The prevalence of Demodex blepharitis in US eye care clinic patients as determined by collarettes: a pathognomonic sign. Clin Ophthalmol. 2022;16:1153-1164.
2. Yeu E, Holdbrook M, Baba SN, et al. Treatment of Demodex blepharitis: a prospective, randomized, controlled, double-masked clinical trial comparing topical lotilaner ophthalmic solution, 0.25% eyedrops to vehicle. Ocul Immunol Inflamm. 2022:1-9.
3. Yeu E, Wirta DL, Karpecki Pet al; Saturn I Study Group. Lotilaner ophthalmic solution, 0.25%, for the treatment of demodex blepharitis: results of a prospective, randomized, vehicle-controlled, double-masked, pivotal trial (Saturn-1). Cornea. 2023;42(4):435-443.
