Dry eye disease (DED) is a multifactorial ocular surface disease that can cause vision fluctuation, pain and discomfort, and inflammation, and in moderate-to-severe cases, it has been shown to cause a significant decrease in patients’ quality of life.1 The hallmark of DED is a loss of tear film homeostasis,2 which can be triggered by numerous factors, including lifestyle, environment, and underlying pathology. Millions of Americans are affected, but only a fraction are treated with prescription medication. Cynthia Matossian, MD, FACS, and Jai G. Parekh, MD, MBA, FAAO, hosted a recent webinar discussing approaches to identifying, evaluating, and diagnosing DED.
Prevalence
DED is a common disorder, yet diagnosis is often missed. An estimated 38 million Americans have signs and symptoms of DED;3 however, only 18 million are diagnosed,3 and a mere 1.1 million are treated with prescription medication.4
Left unchecked, DED can result in significant patient impairment.1 Excessive tear evaporation and instability lead to a cycle of desiccation stress, ocular surface inflammation, and dry eye signs and symptoms.5 Insufficient tear quantity,2 lipid deficiency,2 and lifestyle and environmental factors6 can all contribute to a tear evaporation rate that exceeds supply (Figure 1). That stress can create permanent tissue damage.5 Fortunately, depending on when it’s caught, we may be able to turn it around.
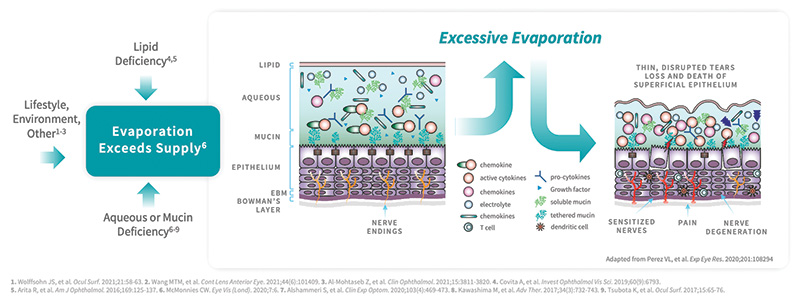
Figure 1. Multiple factors contribute to excessive tear evaporation in DED.
Approaches to Diagnosis
There is a huge discrepancy between patients diagnosed and patients who are being treated. The reasons for this are manyfold: some patients do not follow through with filling prescriptions, their physicians do not feel that treatment of dry eye is warranted, or symptoms may be dismissed. “This is why DED patients are going from doctor to doctor to doctor looking for one who will first listen and believe what they say, and then appropriately make the diagnosis and implement a treatment algorithm. Diagnosing DED can be done easily and does not require sophisticated, costly equipment. There are several methods we can employ to evaluate our patients for DED,” said Dr. Matossian.
Subjective Questionnaires
Validated questionnaires, such as the Ocular Surface Disease Index (OSDI)7 and Standard Patient Evaluation of Eye Dryness (SPEED) Score,8 are subjective questionnaires answered by patients and can be an excellent source of information (Figure 2). However, these may confuse some patients, and in a busy practice may prove time consuming and impractical. “We have taken pieces of each and empowered our technicians to directly ask patients the following three questions: 1) Does your vision change throughout the day, and if so, what time of day is it the worst? 2) How many times a day do you use artificial tears? And, 3) Do your eyes feel tired and do you want to close them? Some practices email the questionnaires out to be completed by the patient prior to their visit,” said Dr. Matossian.
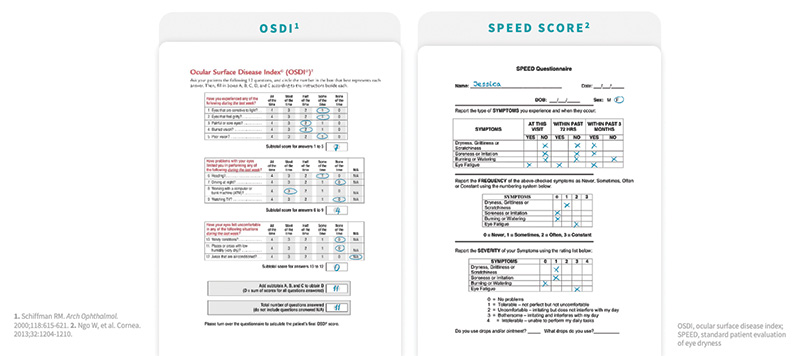
Figure 2. In this example patients, a score of 25 on the OSDI (left) and a score of 8 on SPEED (right) each indicate very mild disease.
There is often a disconnect between symptomology and what we see during the slit-lamp exam, which can make diagnosis challenging. This mismatch may be attributed to several factors, such as chronic pain, atopic disease, and depression.9 “Patients with lower self-perceived overall health report more severe symptoms versus signs. We need to look at the patient, hear what they are saying, ask about fluctuating vision, and measure it. We are likely to stumble upon dry eye either directly or indirectly,” said Dr. Matossian.
Objective Measures of Tear Film Stability
Objective measurements are also available. “Patients like numbers, so we use tear osmolarity and MMP-9. With these tests, we can see the full story plus what we see clinically and symptomatically. We test before all intraocular surgeries, as well as once or twice throughout the year. I share the numbers with the patients, especially asymptomatic patients, so that they believe there is DED and know what is going on. These numbers are also helpful for monitoring therapeutic treatment effects. Patients tend to be more motivated if they see their numbers getting better,” said Dr. Parekh.
Dry eye is also visible with staining. “When staining, I use strips to avoid overwhelming the eye surface with a lot of liquid. I pull the lid down, put a little bit of the strip inside the cul-de-sac and then have the patient blink a few times. My preferred stain is fluorescein, which will show desiccation and tells me there is DED (Figure 3). If the cornea is pristine and I suspect an issue with the ocular surface, I will use lissamine green to stain the conjunctiva to better show the nature of the ocular surface,” Dr. Parekh continued.

Figure 3. Inferior corneal fluorescein staining indicative of DED.
Assessing Meibomian Gland Function
Evaporative tear loss often overlaps with aqueous deficiency and is the underlying etiology in nearly 90% of DED cases.10 Meibomian gland dysfunction (MGD) is a principal contributor to evaporative DED.2 If the meibum secreted is reduced or altered, tears lose their aqueous-protecting lipid layer, leading to a cycle of more rapid tear film disruption and evaporation.11 “The worse the MGD, the thinner the lipid layer is. Evaporation and MGD make up a vicious cycle that continues like two cyclones,” said Dr. Matossian.
Evaluating meibomian gland function is an essential part of diagnosing dry eye. “You cannot tailor your treatment unless you know the etiology of patients’ dry eye. Assessing for MGD is quick and can be done with a slit lamp. ASCRS now uses the acronym LLPP—look, lift, push, pull. Look for telangiectatic vessels at the edge of the lid margin, lash misdirection, and ‘slippery’ lids. Pull the lid down, lift the lid up, and poke at the meibomian glands using a cotton swab, your finger, or a spatulated instrument. Caps that look ‘buttery’ are a definite indication of blockage and may be unplugged using pressure if caught early. Older occlusions may require more aggressive measures,” said Dr. Parekh.
“For a more objective approach to diagnosis, meibomian gland imaging is a powerful diagnostic tool that literally shows us MGD in black and white. We can use these images to determine a meiboscore. Graded from zero to 3, the meiboscore number increases with telltale signs of gland loss, alterations, and changes to architecture,” said Dr. Matossian.
Addressing Evaporation, the Final Common Pathway in DED
DED causes damage to the cornea and can negatively impact visual function and quality of life. “Some existing prescription DED treatments are associated with high rates of dissatisfaction and discontinuation.12 Preservative-free artificial tears are typically my first line of treatment; however, residence time is brief, and thicker drops are known to cause blurred vision and reduce contrast sensitivity,”13 said Dr. Parekh.
“We can intervene in MGD with a combination of home therapies, such as microwavable masks and lens cleansers, and by using lid-warming devices and decompression in clinic. However, there is currently not an FDA-approved prescription eye drop to target evaporation, the underlying mechanism in DED,” said Dr. Matossian.
©2023 Bausch & Lomb Incorporated or its affiliates. UBL.0019.USA.23
1. Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep. 2013;1(2):51-57.
2. McMonnies CW. Aqueous deficiency is a contributor to evaporation-related dry eye disease. Eye and Vis (Lond). 2020;7:6.
3. 2020 Dry Eye Products Market Report: A global Analysis for 2019 to 2025. Market Scope. https://www.market-scope.com/pages/reports/250/2020-ophthalmic-landscape-report-global-analysis-for-2019-to-2025-april-2021#reports
4. IQVIA data.
5. Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: What we know and future directions for research. Ophthalmology. 2017;124(11S):S4-S13.
6. Wolffsohn JS, Wang MTM, Vidal-Rohr M, et al. Demographic and lifestyle risk factors of dry eye disease subtypes: a cross-sectional study. Ocul Surf. 2021;21:58-63.
7. Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615-621.
8. Ngo W, Situ P, Keir N, et al. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea. 2013;32(9):1204-1210.
9. Vehof J, Smitt-Kamminga NS, Nibourg SA, Hammond CJ. Predictors of discordance between symptoms and signs in dry eye disease. Ophthalmology. 2017;124:280-286.
10. Lemp M, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-8.
11. Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: The role of gland dysfunction in dry eye disease. Ophthalmology. 2017;124(11S):S20-S26.
12. White DE, Zhao Y, Ogundele A, et al. Real-world treatment patterns of cyclosporine ophthalmic emulsion and lifitegrast ophthalmic solution among patients with dry eye. Clin Ophthalmol. 2019;13:2285-2292.
13. Garrigue JS, Amrane M, Faure MO, et al. Relevance of lipid-based products in the management of dry eye disease. J Ocul Pharmacol Ther. 2017;33(9):647-661.


