CASE PRESENTATION
A 30-year-old man presents for a refractive surgery evaluation. The patient has no significant medical or ocular history. His manifest refraction is -3.25 D of sphere OD and -4.00 -0.75 x 010º OS, and his BCVA is 20/20 OU. Imaging with the Pentacam (Oculus Optikgeräte, Figure 1) is reassuring, and he undergoes uncomplicated bilateral SMILE.
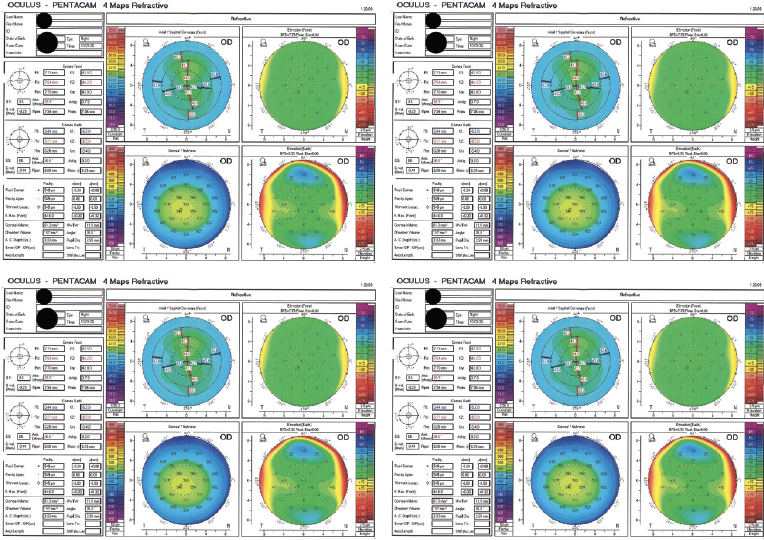
Figure 1. Imaging with the Pentacam before SMILE.
One week after surgery, the patient’s BCVA is 20/20 OD with a plano refraction and 20/20 OS with a manifest refraction of -0.50 -1.00 x 175º. The ocular surface of each eye is healthy, and the remainder of the examination is unremarkable. Six months after SMILE, the refraction and visual acuity are stable in each eye (Figure 2).
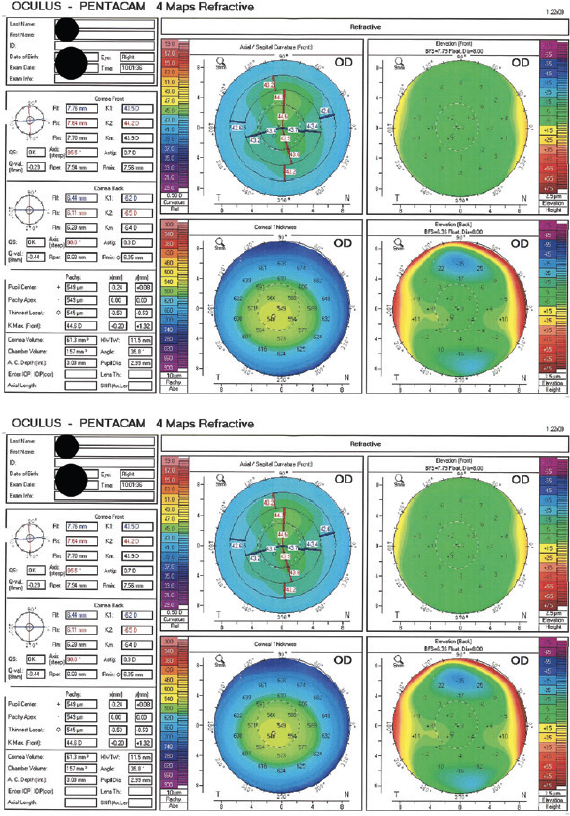
Figure 2. Imaging with the Pentacam 6 months after SMILE.
The patient expresses a desire for a refractive enhancement in the left eye, which has a pachymetry reading of 470 µm.
How would you counsel the patient? What surgical intervention would you recommend?
—Case prepared by Neel Vaidya, MD, MPH; Parag Majmudar, MD; Arjan Hura, MD; and William F. Wiley, MD

JOHN F. DOANE, MD, FACS
The left eye has residual myopic astigmatism. Astigmatic correction with SMILE is highly accurate overall, but as can occur with excimer laser treatment, there are instances when initial surgery does not hit the target.
Pre- and postoperative imaging with the Pentacam is within normal limits. The 80-µm difference between the pre- and postoperative pachymetry readings is excessive for a refractive change of 3.37 D, but it could be within the error range of ultrasound pachymetry. I have found OCT to be more accurate and precise than ultrasound.
The good news for the patient is that his BCVA is excellent, so an enhancement with corneal refractive surgery is an option. Given the residual refractive error, I would favor performing a pseudo-Circle enhancement using the VisuMax femtosecond laser (Carl Zeiss Meditec) in which a LASIK flap is created from the SMILE cap by using a 300º vertical photodisruption pattern and then excimer laser ablation to resolve the residual refractive error. See video below for a demonstration. (Editor’s note: Any form of surgical enhancement of a primary laser vision correction procedure is off label.)

KATHRYN M. HATCH, MD
I would first confirm that the patient believes he requires surgical intervention. Not all patients are bothered by mild residual ametropia, and the goal of surgery should be to achieve the patient’s desired outcome.
Assuming a refractive enhancement is desired, I would obtain epithelial thickness mapping because epithelial remodeling after refractive surgery plays a key role in healing and the final refractive outcome. The results of this test are a major factor in the decision between two enhancement techniques: PRK and the Circle technique that Dr. Doane described in his response. If the epithelial thickness map results show a diffusely thickened, hypertrophic epithelium, PRK is likely to be ineffective and produce an erroneous or unpredictable result owing to unpredictable epithelial remodeling. If, however, the thickness of the epithelium is uniformly normal, PRK is a reasonable approach.
If PRK is planned, it would be important to discuss postoperative recovery with the patient in advance and to administer mitomycin C for at least 30 seconds during the procedure to reduce the risk of postoperative haze.

PAVEL STODULKA, MD, PHD
There are several options for fixing a residual refractive error after SMILE. Because the patient’s error is too low for correction by repeat SMILE or the placement of a phakic IOL, the options are the Circle technique, thin-flap LASIK, and surface ablation.
The thickness of the cap is not specified in the case presentation, but it typically ranges from 130 to 160 µm. Enough tissue in a 470-µm cornea should therefore remain to permit either thin-flap LASIK or surface ablation to be performed safely. That said, creating a LASIK flap and performing excimer laser ablation would be tight if a 130-µm cap was created during SMILE.
I usually create a 160-µm cap, and I tend to perform PRK to correct residual myopia after SMILE. I find surface ablation to be the most straightforward approach to correcting low myopia. Current excimer laser systems create a smooth stromal bed, helping patients to see well during the first 2 days after PRK, although their vision temporarily decreases when reepithelialization reaches the center of the cornea. The main drawback of PRK is pain. With proper pain management, however, surface ablation can be an acceptable option.
Topography-guided treatment can be used to fine-tune the result after all three of the techniques I mentioned, but it is not required here.
Before proceeding with surgery, I would explain to the patient the potential advantages of having a small amount of myopia once presbyopia develops. Based on the information provided, however, he desires emmetropia in both eyes, a goal typical of people his age.


WHAT WE DID: ARJAN HURA, MD, AND WILLIAM F. WILEY, MD
Approaches to addressing a residual refractive error after routine SMILE include PRK, thin-flap (80-µm) LASIK, thick-flap (140-µm) LASIK, and cap-to-flap conversion. Healing after PRK is prolonged, and both thin- and thick-flap LASIK carry a risk of perforating the tissue bridge formed between the LASIK flap and residual SMILE cap.
We elected to perform a cap-to-flap conversion. Circle software (Carl Zeiss Meditec) can be used to convert a SMILE cap to the equivalent of a LASIK flap, but the software is not available in the United States where we practice. Instead, an off-label technique was used to convert the SMILE cap to a LASIK flap. The technique involves starting, stopping, and then restarting flap creation with a VisuMax femtosecond laser.
A 120-µm flap was programmed, which was the same depth as the SMILE cap bed. Suction was established on the eye and intentionally broken after flap creation extended past the edges of the SMILE cap. Flap creation was then restarted with the eye uncoupled from and out of the way of the laser; artificial suction had been established by occluding the vacuum port with a finger. The treatment was interrupted again at 4 seconds, after the flap bed was complete but before the sidecut of the flap was performed. The laser was docked to the eye, and the sidecut was completed. The flap was lifted as a standard LASIK flap would be, but care was taken when drying the bed to avoid creating tissue wafers where the flap intersected with the peripheral cap bed.
The flap treatment overlapped only the periphery of the cap bed. There was no double pass of the femtosecond laser across the visual axis and no chance of creating a corneal wafer in the visual axis. A standard excimer laser ablation enhancement of the flap and cap bed was performed.
One week after surgery, the patient’s BCVA was 20/15 OS with a plano refraction.




