CXL is approved by the FDA for two indications. These are unstable keratoconus and ectasia after refractive surgery. Today, the procedure is a first-line treatment for patients with these conditions, and it dramatically reduces their need for corneal transplantation.1 The FDA-approved protocol (iLink, Glaukos) follows the original epithelium-off (epi-off) CXL Dresden protocol. After the central 8 to 9 mm of corneal epithelium are removed, riboflavin drops are instilled onto the corneal stroma, and UV-A light is administered (Figure 1). The riboflavin activation under UV-A irradiation causes the formation of reactive oxygen species that covalently bond corneal collagen proteins. The result is a biomechanically stiffened cornea that is more resistant to ectatic progression. More than 90% of patients experience stability for 10 years after CXL.2 The procedure can also improve visual acuity and contact lens fit by flattening the cornea.
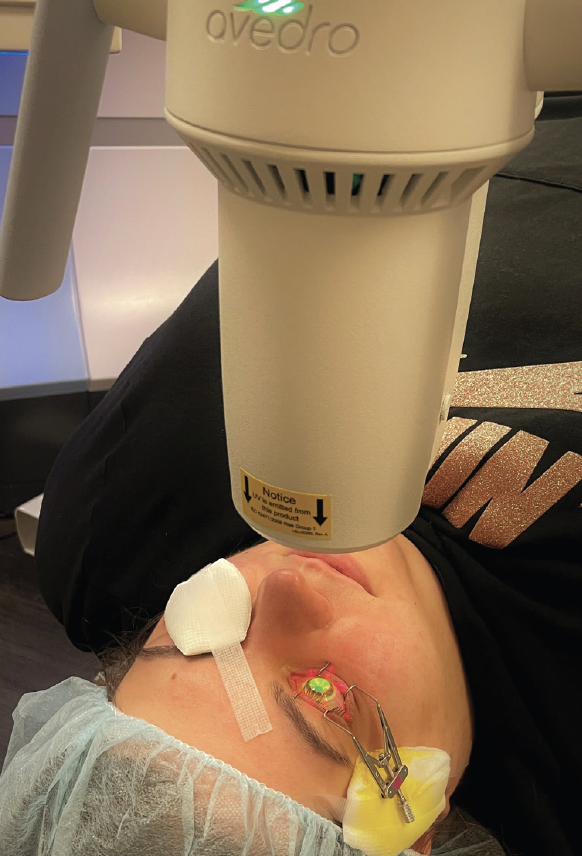
Figure 1. A patient undergoing epi-off conventional CXL in the right eye. The riboflavin-soaked cornea is being irradiated by UV-A light.
Many variations on the conventional CXL protocol are being tested around the world to decrease procedural time (accelerated protocols) and make epithelial removal unnecessary (epithelium-on protocols), but epi-off CXL remains the standard of care in the United States. This article discusses perioperative planning and the management of patients undergoing conventional CXL.
PATIENT SELECTION AND PREOPERATIVE CONSIDERATIONS
Indications. CXL is indicated for any patient with progressive corneal ectasia who does not have a contraindication, such as a history of viral keratitis, poor wound healing, neurotrophic keratitis, and/or limbal stem cell deficiency. The procedure has been used most widely for the treatment of keratoconus, but it has also shown effectiveness for the treatment of ectasia after refractive surgery and pellucid marginal degeneration.3 CXL is effective and safe in children as young as 8 years of age,4 but it is approved in the United States for the treatment of patients who are 14 years of age or older.
Identifying progressive ectasia. A clinical determination of ectatic progression is based on changes in visual acuity, refractive error, and corneal topography. There is not, however, consensus on the exact criteria for determining progression. In the FDA trials of CXL for the treatment of keratoconus, progression was defined as a change in spherical correction of at least 0.50 D, a change in cylinder correction of at least 1.00 D, or a change in the maximum keratometry (K) reading of at least 1.00 D in a 24-month period.5 Most US insurance carriers adhere to these criteria.
The examination. Patients for whom CXL is being considered should receive a thorough eye examination (see A Call for More Aggressive Keratoconus Screening). Manifest refractions, K readings, and contact lens fittings may be compared to patient records to identify changes over time. Scheimpflug imaging can reveal corneal irregularity and steepening. Coexisting ocular disease should be documented and addressed.
A CALL FOR MORE AGGRESSIVE KERATOCONUS SCREENING
Keratoconus progresses and reduces the BCVA of too many people. Eye care providers must become more aggressive about screening for the disease and teaching patients that they can induce keratoconus by repeatedly applying pressure to their eyes.
Parallels may be drawn between glaucoma and keratoconus care. Vision loss from both diseases is preventable. Whereas glaucoma screening is a routine part of an eye exam, keratoconus screening is not. The incidence of vision loss from a delayed diagnosis of keratoconus is high. Too many patients lose BCVA before being asked if they rub their eyes, before they undergo corneal topography, and before genetic screening is considered. (Editor’s note: For more on genetic screening for keratoconus, see “Say Anything.”)
We ask all patients who visit our offices if they rub their eyes—it is a check box on our forms. We have found that patients often do not realize that rubbing their eyes can change their prescription and damage their eyes from front to back. Oftentimes, patients will say that they do not rub their eyes when first asked and then, at a follow-up appointment, state that they rub their eyes more than they realized.
Pachymetry measurements are obtained so that the risk of UV-A light–induced endothelial damage may be assessed. A minimum corneal thickness of 400 µm is recommended for CXL. The application of hypoosmolar riboflavin during the procedure can swell the corneal stroma to achieve this thickness. If the minimum thickness cannot be achieved, as may occur if the ectasia is advanced, we recommend proceeding with CXL because we consider the risk of untreated keratoconus to be greater than the rare risk of endothelial damage from the procedure.
Comorbid ocular surface disease. Corneal ectasia is thought to result from an interplay between aggressive eye rubbing and reduced corneal biomechanical rigidity from a genetic (keratoconus) or iatrogenic (post–refractive surgery) predisposition. Patients with keratoconus also often have atopic ocular surface disease (OSD). Itching and irritation caused by OSD can prompt patients to rub their eyes, producing IOP spikes upwards of 300 mm Hg.6 The tremendous stress on the cornea can cause a cone to form. It is therefore extremely important to control symptoms of atopic OSD and dry eye disease (DED) in patients with evidence of ectasia regardless of whether they are candidates for CXL. Atopic eye disease may be treated with topical and/or oral allergy medications in addition to topical steroids or immunomodulators as needed. The aggressive treatment of DED may require the use of artificial tears, ointments, and/or punctal plugs.
Behavioral assessment. As mentioned earlier, eye rubbing plays a role in ectatic progression, so ectasia can recur after CXL if this behavior is not controlled. It should also be noted that patients may rub their eyes while sleeping. Another behavioral cause of cone formation is sleeping facedown with resultant pillow or hand pressure on the eyes at night. It therefore may be beneficial for patients with progressive corneal ectasia to wear protective eye masks or goggles while sleeping.
POSTOPERATIVE MANAGEMENT
Because the epithelium is removed during conventional CXL, immediate postoperative care focuses on pain control, infection prevention, and epithelial wound healing. Most providers place a bandage contact lens on the eye at the end of the procedure to improve patient comfort. Some prescribe topical NSAIDs for their analgesic effects. Oral analgesics include over-the-counter options such as acetaminophen and, less commonly, prescription opioids. The safety of topical anesthetics after CXL remains controversial, but some providers allow patients to use these agents sparingly during the first 48 to 72 hours after surgery.
Topical antibiotics are indicated in all cases. A third-generation fluoroquinolone is recommended to broadly cover the most common periocular flora. In certain situations, oral doxycycline, azithromycin, or hypochlorous acid spray may be used adjunctively to decrease the periocular microbial load. Topical steroids are administered to reduce postprocedural inflammation and the risk of corneal scarring and haze. Some providers wait 4 to 5 days until the epithelial defect has closed before starting steroids to reduce the risk of infection. The risk of sterile infiltrate formation, however, is higher during this period if steroids are not used, which may have a lasting visual impact.7 For this reason, we recommend initiating treatment with topical steroids immediately after CXL. Patients should be reminded not to rub their eyes and instructed to wear protective goggles at night for the first week.
Patients require close observation during the immediate postoperative period to ensure that the epithelium closes without complication. The bandage contact lens is removed within the first week. Topical antibiotics may be discontinued once the epithelium heals and the contact lens is removed. Topical steroids are tapered over 2 to 4 weeks.
COMPLICATIONS
Corneal haze and scarring. Corneal haze is the most common complication after CXL. In an FDA trial, 57% of patients with keratoconus and 68% with post–refractive surgery ectasia exhibited corneal haze 1 month after CXL that resolved by 1 year in most cases.5,8 Corneal scarring occurs much less frequently than corneal haze.2 Both complications are more common and tend to be more severe in patients with advanced keratoconus,9 but these individuals also generally experience the greatest degree of corneal flattening after CXL. This suggests that haze formation is directly related to the degree of corneal remodeling that occurs after CXL.
Infectious keratitis. This complication, though rare, can be devastating. Most instances after CXL are secondary to gram-positive bacterial infection and occur within the first week after surgery, but fungal and viral infections also occur.9 A history of OSD may predispose patients to infection, especially those who have a history of long-term use of topical antibiotics or steroids. The organisms cultured from these infections are more likely to show antibiotic resistance. Patients with atopic keratitis therefore may benefit from a povidone-iodine preparation before removal of the epithelium.
Delayed wound healing. Problems with wound healing are usually caused by undiagnosed or undertreated OSD. Delayed wound healing is also more likely in patients who have a history of limbal stem cell or neurotrophic pathologies such as diabetes, herpetic infection, or autoimmune disorders. Poor wound healing can itself be an early sign of herpetic infection. Aggressive ocular lubrication and/or the extended use of a bandage contact lens may be sufficient to encourage the epithelium to close. More severe cases may require amniotic membrane transplantation or tarsorrhaphy. Antivirals may be started if herpetic infection is a concern.
Disease progression. Keratoconus progresses rarely after CXL. This complication is more likely among children, patients with atopic disease, and individuals who chronically rub their eyes.10 Repeat CXL may be indicated in this situation. As noted earlier, patients should be monitored closely for disease progression during the first year after CXL—usually at 1, 3, 6, and 12 months—and annually thereafter.
PATIENT EXPECTATIONS
After CXL, the corneal stroma thins as bonds form between the collagen fibrils. Pachymetry readings thus decrease initially after treatment but rebound nearly to preoperative levels during the first year (Figure 2).1 The same trend is observed with K readings. One to 3 months after CXL, the cornea is thinner and steeper than before treatment. A flattening effect typically starts at around 3 to 6 months and may continue for up to 10 years, with K measurements decreasing by 1.00 to 2.00 D on average within the first year.5,11,12 Flattening is thought to be caused by regularization of the collagen layers.
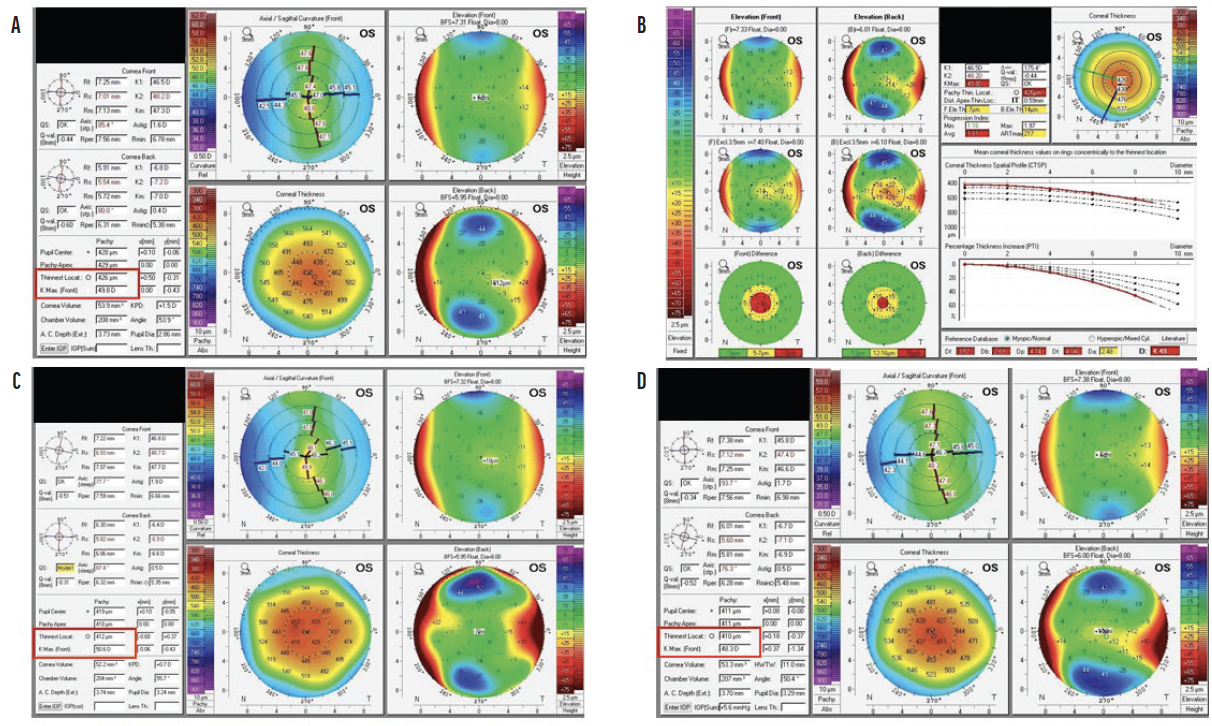
Figure 2. Scheimpflug images (Pentacam, Oculus Optikgeräte) obtained preoperatively (A and B) and 2 months (C) and 6 months (D) after CXL. The Kmax reading increased over the first 2 to 3 months, and a flattening effect was observed at 6 months. Corneal thickness decreased after CXL.
Improved visual acuity is not a primary outcome of CXL, but regularization of the collagen layers often leads to moderate improvement—an effect that is more pronounced in patients with more severe disease before surgery.13 It is important to inform patients preoperatively that their glasses and contact lens prescriptions are likely to change after CXL and continue to change over the next few years. Depending on the severity of preoperative ectasia, patients may require hard contact lenses or scleral lenses to maximize their BCVA after CXL.
1. Sarezky D, Orlin SE, Pan W, VanderBeek BL. Trends in corneal transplantation in keratoconus. Cornea. 2017;36(2):131-137.
2. Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg. 2015;41(1):41-46.
3. Van Tigchelt L, Van Eijgen J, Delbeke H. Alternative indications for corneal crosslinking. J Cataract Refract Surg. 2021;47(10):1360-1366.
4. Padmanabhan P, Rachapalle Reddi S, Rajagopal R, et al. Corneal collagen cross-linking for keratoconus in pediatric patients-long-term results. Cornea. 2017;36(2):138-143.
5. Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK; United States Crosslinking Study Group. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology. 2017;124(9):1259-1270.
6. Turner DC, Girkin CA, Downs JC. The magnitude of intraocular pressure elevation associated with eye rubbing. Ophthalmology. 2019;126(1):171-172.
7. Teal P, Breslin C, Arshinoff S, Edmison D. Corneal subepithelial infiltrates following excimer laser photorefractive keratectomy. J Cataract Refract Surg. 1995;21(5):516-518.
8. Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK; United Statets Crosslinking Study Group. U.S. multicenter clinical trial of corneal collagen crosslinking for treatment of corneal ectasia after refractive surgery. Ophthalmology. 2017;124(10):1475-1484.
9. Kim BZ, Jordan CA, McGhee CN, Patel DV. Natural history of corneal haze after corneal collagen crosslinking in keratoconus using Scheimpflug analysis. J Cataract Refract Surg. 2016;42(7):1053-1059.
10. Lenk J, Herber R, Oswald C, Spoerl E, Pillunat LE, Raiskup F. Risk factors for progression of keratoconus and failure rate after corneal cross-linking. J Refract Surg. 2021;37(12):816-823.
11. Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008;34(5):796-801.
12. Greenstein SA, Hersh PS. Corneal crosslinking for progressive keratoconus and corneal ectasia: summary of US multicenter and subgroup clinical trials. Transl Vis Sci Technol. 2021;10(5):13.
13. Pasha H, Palazzolo L, Prakash G, Jhanji V. Update on corneal collagen crosslinking for ectasia. Curr Opin Ophthalmol. 2021;32(4):343-347.