This content is for U.S. healthcare professionals only.
Indications and Usage
DURYSTA™ (bimatoprost intracameral implant) is indicated for the reduction of intraocular pressure (IOP) in patients with open angle glaucoma (OAG) or ocular hypertension (OHT).
INTRODUCTION
DURYSTA (Allergan, an AbbVie company) is the first FDA-approved, biodegradable intracameral, sustained-release implant indicated to reduce intraocular pressure (IOP) in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT).1 DURYSTA is an interventional therapy that represents a paradigm shift in how IOP-lowering medicines are delivered.
The ARTEMIS 1 and ARTEMIS 2 phase 3 clinical trials were 2 multicenter, randomized, parallel-group, controlled studies comparing the 10-mcg bimatoprost implant to twice-daily timolol 0.5% topical drops. Parallel groups of patients diagnosed with OAG or OHT (with a baseline IOP of 22 to 32 mm Hg at hour 0 after washout) were followed for a period of 20 months, including an 8-month extended follow-up. DURYSTA lowered mean IOP by up to 33% from baseline over the 12-week primary efficacy period. This works out to be about 5 to 8 mm Hg of reduction from the mean baseline IOP of 24.5 mm Hg (Figure 1).1,2
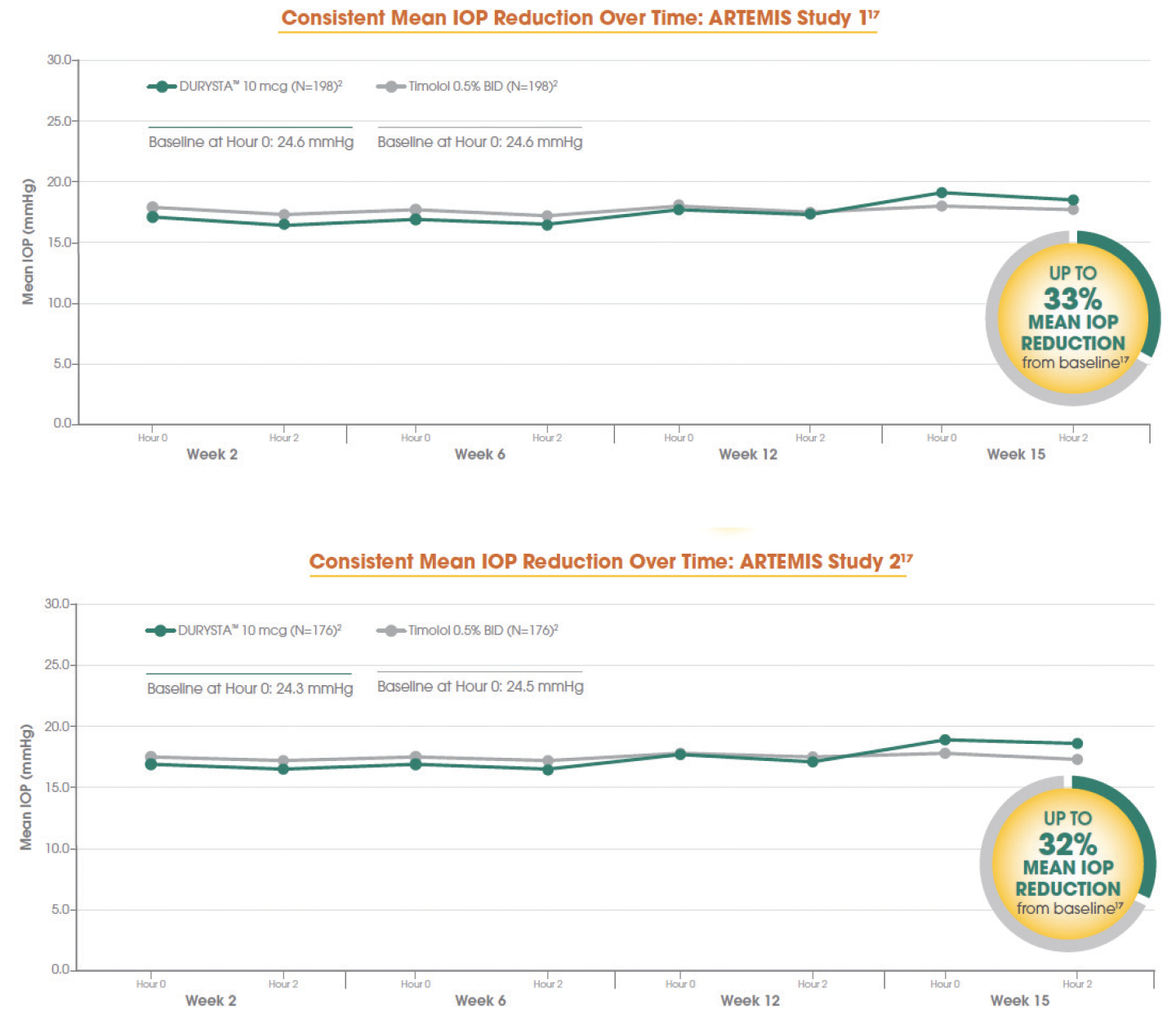
Figure 1. Data from the ARTEMIS 1 Trial (top) and the ARTEMIS 2 Trial (bottom).
In this, the second installment in a 3-part series, Arsham Sheybani, MD, spoke with Preeya Gupta, MD; Manjool Shah, MD; Jacob Brubaker, MD; and George Tanaka, MD. These ophthalmologists gathered to discuss how they foresee implementing DURYSTA into their treatment plans and clinic flow.
PATIENT SCREENING
Arsham Sheybani, MD: Whenever we have an opportunity to usher in a therapeutic modality, we must also work through the optimal means to integrate it into our individual practices. In the first installment of this DURYSTA educational series, we discussed a variety of patients that would benefit from this biodegradable, sustained-release implant. Here, we want to discuss the considerations when offering this treatment and patient selection. There are certain anatomical considerations that should be taken into account. DURYSTA should be used with caution in patients with narrow iridocorneal angles or anatomical obstruction that may prohibit settling in the inferior angle. What are some other factors we should be screening for?
Jacob Brubaker, MD: DURYSTA is contraindicated in patients with corneal endothelial cell dystrophy or prior corneal or endothelial cell transplants. It is also contraindicated in patients whose posterior lens capsules are absent or ruptured due to the risk of implant migration into the posterior segment.1 It is important to properly screen patients and identify the markers that may indicate a patient is at higher risk for complications prior to presenting DURYSTA as an option. Also, DURYSTA should not be administered in any eye that has already received DURYSTA.
Preeya Gupta, MD: Fortunately, we have many methods to assess corneal health. Virtually every practice has access to a slit lamp where we can look for indicators of Fuchs’ dystrophy, such as guttata or stress lines. In patients with a tube, signs of early endothelial dysfunction may include vague, horizontal oblique lines along the posterior cornea and through the posterior stroma and Descemet’s membrane. Gonioscopic grading according to the Shaffer scale is also helpful for screening patients, and pachymetry is another easy test that most glaucoma practices have available.
The following sections discuss administration of DURYSTA. For complete administration instructions, please refer to the accompanying full Prescribing Information.
WHERE TO ADMINISTER DURYSTA
Dr. Sheybani: Let’s talk about clinic flow. We have a lot of options for how we can implement DURYSTA into our practices. What are your thoughts on how you will implement DURYSTA into your individual clinics?
Dr. Brubaker: For the phase 3 study, we performed it in our minor procedure room. There is, also, the option of doing it at an ambulatory surgical center (ASC), if that makes sense for you.
Dr. Gupta: Whether administering at our practice or an ASC, efficiency is an important consideration. Engaging staff is key, and I make it a point to involve them in creating best practices. When implementing procedures, having a procedural protocol in place from start to finish—from the sterilization routine to postprocedure communication to educating staff on warning signs and symptoms—helps ensure things run smoothly. Training and cross-training staff members on what you need and how to prepare the patient is probably the most important aspect of achieving a good flow.
George Tanaka, MD: Assigning responsibilities to the technicians—such as setting up the applicator and tools, prepping the patient, helping to stabilize the head, comforting the patient, etc.—can really help to maximize your time. If you build up volume, you might consider a dedicated technician and room for DURYSTA. There are a lot of options on how to implement this into the flow of your clinic. You can schedule all the administrations together within specific time blocks or incorporate them into the routine practice. My preference is to intersperse the appointments, so I can go back and forth between patients while the technician is gathering supplies and prepping the room and patient for the procedure.
PROPHYLAXIS
Dr. Sheybani: While the clinical trials had no reported incidences of endophthalmitis, these types of intraocular procedures and injections have been associated with endophthalmitis. Proper aseptic technique should be used, and patients should be monitored following administration. How will you handle this?
Dr. Brubaker: From an infection prophylaxis standpoint, povidone iodine is one of the most effective molecules we have. I instill 1 or 2 drops from a vial of premixed ophthalmic solution (0.5% concentration) in the cul-de-sac and have the patient blink a few times. Blinking causes the povidone iodine to touch the lashes, where a lot of the bacteria live. To keep the DURYSTA applicator sterile, I have the technician open the package up, and then I take it right out of the pouch, and remove the pin and cap.
Dr. Tanaka: If the doctor suspects that a patient has a higher risk of infection than the average population (eg, nursing home patients, those with severe blepharitis, patients with circumstances that cause them to touch their eyes), it is their discretion as to whether they want to consider an antibiotic. Similar to other intraocular procedures, patients should be monitored following administration of DURYSTA.1
The following sections discuss administration techniques. Some of the opinions in this section are not found within the full Prescribing Information and are based on the physician's own clinical experience.
ADMINISTRATION TECHNIQUE
Dr. Sheybani: In the phase 3 trial, DURYSTA was studied with patients in the supine position. The label indicates that, when administering DURYSTA, there must be magnification that allows clear visualization of the anterior chamber structures, standard aseptic conditions, and the patient’s head must be in a stabilized position.1 When learning a procedure, it is always helpful to hear how our colleagues are doing this. Can you each share your perspective on how you are performing the administration of DURYSTA?
Manjool Shah, MD: As the label indicates, we want to stabilize the patient’s head and ideally the eye, while performing the procedure under magnification.1 In my experience, one pearl I discovered is to tunnel up toward the apex of the cornea by a half millimeter or a millimeter, prior to entering the anterior chamber. That little bit of tunnel and beveled entry help to keep the anterior chamber stable and facilitate delivery of the implant. Since DURYSTA is administered using a fine 28-gauge needle,3 topical anesthesia is adequate for most patients.
Dr. Tanaka: In addition to topical anesthetic, I like to place a sterile cotton swab dipped in 4% lidocaine right over my injection site to numb the cornea. The applicator needle creates some resistance as it is entering, so I find a cotton swab very useful for counter-traction. Patients just need to be able to fixate on a target. In patients who are extremely anxious, I may consider administering a sedative to help them relax.
Dr. Gupta: Upon entering the eye, we must be mindful of the anterior capsule. If you feel needle resistance, then you have not gone far enough into the eye. Once the resistance disappears, you are into the anterior chamber. You want to make sure that you are not intrastromal. In fact, you want to be ≈ 2 bevel lengths into the anterior chamber to ensure the implant is cast far away from the needle tip, so it will not be drawn back to the corneal incision.
ADMINISTRATION PEARLS
Dr. Sheybani: With any procedure, it requires experience to internalize the nuances and techniques. From your clinical trial experience administering DURYSTA, what particulars and pearls can you share with our colleagues regarding application and administration techniques?
Dr. Gupta: Stabilization is very important. To stabilize the eye, I apply a little counter-traction using a soft cotton swab positioned 180° away from where I want to make my incision. This provides enough force for entry into the anterior chamber so that the hub is not left in the stroma. I also stabilize part of my hand on the side of the patient’s face. This gives me a sense of whether the patient is moving before he or she has a manifest movement.
Dr. Brubaker: I recommend removing the cap and safety tab from the applicator yourself. You do not want the risk of your staff dinging the 28-gauge needle and then possibly entering the cornea with a burr on the applicator. Make sure that you are familiar with the applicator and practice holding and deploying it. Also make sure your thumb is in the right place so that you can easily find the deployment button and minimize the amount of time you are in the eye, as well as needle movement.
Dr. Shah: It’s very important to be decisive and quick when depressing the button to ensure the implant is expelled from the applicator. If you do a light, gentle click, there is a greater likelihood that the implant will stick on the end of the needle. To ensure nice entry architecture, I always face the bevel interior toward me so I can confirm that I am ≈ 2 bevel lengths into the anterior chamber. To achieve the required delivery force, depress the back half of the actuator button until it clicks. The needle has a little bit of flexibility to it. To avoid any inadvertent bend in the needle that could hinder the deployment mechanism, I bolster my treating hand on the side of the patient’s forehead or cheek and ensure that the needle is axial and without any bend or bias. I also recommend that you remove the injector and needle from the anterior chamber decisively with a slight anterior bias in order to help self-seal the corneal track.
POSTADMINISTRATION PROCEDURES
Dr. Sheybani: The DURYSTA Prescribing Information instructs us to have the patient in an upright position for at least 1 hour after insertion so that the biodegradable implant can settle into the inferior position.1 In my practice, I will consider using a gel tear in the eye to mitigate any povidone iodine sensitivity and then have the patient sit upright in the waiting room with the eye closed for an hour. How long do you think is necessary to keep the patient, and when will you bring the patient back?
Dr. Tanaka: As part of the study, we would have patients sit upright and check right away to verify that the implant settled down into the right spot. We had them stay on-site for an hour and then checked them again. It is the physician’s discretion as to how long they wait before allowing the patient to leave, but they should instruct their patient to remain upright for at least 1 hour post administration.
Dr. Shah: I concur that it is up to the physician how long they want to monitor the patient before allowing them to leave. As the implant settles in quickly, I do not place any further restrictions on them and just suggest they take it easy for the day. Inform your patients to expect some degree of eye redness and discomfort and to contact you immediately if they experience any side effects including progressive redness, light sensitivity, pain, or visual changes after the injection.1
Dr. Brubaker: I think a check for tolerance and pressure at the 2-week mark is sufficient. If they achieve the desired eye pressure response, this may be a good opportunity to treat the second eye. After that, I think it is appropriate to see them as you see fit.
Some of the opinions in this section are not found within the Prescribing Information and are based on the physician’s own clinical experience.
TROUBLESHOOTING
Dr. Sheybani: Although administering DURYSTA is straightforward, it requires delicate and precise administration. Have you experienced any problems during administration, and, if so, how did you resolve them?
Dr. Brubaker: Make sure that you are pressing on the applicator with enough force that the implant is released from the applicator. If the implant does remain in contact with the administration track, it should not be allowed to remain there. Try to gently tap the cornea over the administration track to cause the implant to drop down. If it remains stuck, you can use an anterior chamber probe to dislodge the implant from the administration track by advancing ≈ 2 to 3 mm into the anterior chamber (Figure 2). This probe can be provided to you by your Allergan representative.

Figure 2. An anterior chamber probe can be used to dislodge the implant from the administration track.
Dr. Tanaka: Occasionally, the implant may adhere to the needle tip. Wait 5 seconds to see if it will disengage on its own. If not, gently press the implant against the anterior iris to engage it and slightly rotate the applicator to disconnect the implant from the needle tip. If that doesn’t work, withdraw the needle from the anterior chamber. Contact with the inner lip of the administration track may release the implant from the needle tip.
Dr. Shah: If you see the implant floating in the anterior chamber, wait and allow it to settle into the inferior position without manipulating it. If it is floating because of an air bubble, the bubble should dissipate on its own.
Dr. Sheybani: Integrating this novel interventional therapy benefits both the patient and the ophthalmologist treating their disease. Thank you for the excellent conversation and your insights, regarding this exciting breakthrough in IOP lowering for the management of glaucoma.
DURYSTA™ Indications and Usage and Important Safety Information
Indications and Usage
DURYSTA™ (bimatoprost intracameral implant) is indicated for the reduction of intraocular pressure (IOP) in patients with open angle glaucoma (OAG) or ocular hypertension (OHT).
Important Safety Information
Contraindications
DURYSTA™ is contraindicated in patients with: active or suspected ocular or periocular infections; corneal endothelial cell dystrophy (e.g., Fuchs’ Dystrophy); prior corneal transplantation or endothelial cell transplants (e.g., Descemet’s Stripping Automated Endothelial Keratoplasty [DSAEK]); absent or ruptured posterior lens capsule, due to the risk of implant migration into the posterior segment; hypersensitivity to bimatoprost or to any other components of the product.
Warnings and Precautions
The presence of DURYSTA™ implants has been associated with corneal adverse reactions and increased risk of corneal endothelial cell loss. Administration of DURYSTA™ should be limited to a single implant per eye without retreatment. Caution should be used when prescribing DURYSTA™ in patients with limited corneal endothelial cell reserve.
DURYSTA™ should be used with caution in patients with narrow iridocorneal angles (Shaffer grade ˂ 3) or anatomical obstruction (e.g., scarring) that may prohibit settling in the inferior angle.
Macular edema, including cystoid macular edema, has been reported during treatment with ophthalmic bimatoprost, including DURYSTA™ intracameral implant. DURYSTA™ should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Prostaglandin analogs, including DURYSTA™, have been reported to cause intraocular inflammation. DURYSTA™ should be used with caution in patients with active intraocular inflammation (e.g., uveitis) because the inflammation may be exacerbated.
Ophthalmic bimatoprost, including DURYSTA™ intracameral implant, has been reported to cause changes to pigmented tissues, such as increased pigmentation of the iris. Pigmentation of the iris is likely to be permanent. Patients who receive treatment should be informed of the possibility of increased pigmentation. While treatment with DURYSTA™ can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly.
Intraocular surgical procedures and injections have been associated with endophthalmitis. Proper aseptic technique must always be used with administering DURYSTA™, and patients should be monitored following the administration.
Adverse Reactions
In controlled studies, the most common ocular adverse reaction reported by 27% of patients was conjunctival hyperemia. Other common adverse reactions reported in 5% 10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, intraocular pressure increased, corneal endothelial cell loss, vision blurred, iritis, and headache.
Please see full Prescribing Information.
© 2021 AbbVie. All rights reserved. DURYSTA™ and its design are trademarks of Allergan, Inc., an AbbVie company. DurystaHCP.com DUR146661 05/21
1. DURYSTA™ Prescribing Information.
2. Data on file, Allergan.
3. Medeiros FA, Walters TR, Kolko M, et al. ARTEMIS 1 Study Group. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1) [published online ahead of press June 13, 2020]. Ophthalmology. doi: 10.1016/j.ophtha.2020.06.018.
DURYSTA™ Important Safety Information for US Healthcare Professionals
Collapse -
Contraindications
DURYSTA™ is contraindicated in patients with: active or suspected ocular or periocular infections; corneal endothelial cell dystrophy (e.g., Fuchs’ Dystrophy); prior corneal transplantation or endothelial cell transplants (e.g., Descemet’s Stripping Automated Endothelial Keratoplasty [DSAEK]); absent or ruptured posterior lens capsule, due to the risk of implant migration into the posterior segment; hypersensitivity to bimatoprost or to any other components of the product.
Warnings and Precautions
The presence of DURYSTA™ implants has been associated with corneal adverse reactions and increased risk of corneal endothelial cell loss. Administration of DURYSTA™ should be limited to a single implant per eye without retreatment. Caution should be used when prescribing DURYSTA™ in patients with limited corneal endothelial cell reserve.
DURYSTA™ should be used with caution in patients with narrow iridocorneal angles (Shaffer grade ˂ 3) or anatomical obstruction (e.g., scarring) that may prohibit settling in the inferior angle.
Macular edema, including cystoid macular edema, has been reported during treatment with ophthalmic bimatoprost, including DURYSTA™ intracameral implant. DURYSTA™ should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Prostaglandin analogs, including DURYSTA™, have been reported to cause intraocular inflammation. DURYSTA™ should be used with caution in patients with active intraocular inflammation (e.g., uveitis) because the inflammation may be exacerbated.
Ophthalmic bimatoprost, including DURYSTA™ intracameral implant, has been reported to cause changes to pigmented tissues, such as increased pigmentation of the iris. Pigmentation of the iris is likely to be permanent. Patients who receive treatment should be informed of the possibility of increased pigmentation. While treatment with DURYSTA™ can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly.
Intraocular surgical procedures and injections have been associated with endophthalmitis. Proper aseptic technique must always be used with administering DURYSTA™, and patients should be monitored following the administration.
Adverse Reactions
In controlled studies, the most common ocular adverse reaction reported by 27% of patients was conjunctival hyperemia. Other common adverse reactions reported in 5% 10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, intraocular pressure increased, corneal endothelial cell loss, vision blurred, iritis, and headache.
Please see full Prescribing Information.








