This content is for U.S. healthcare professionals only.
Indications and Usage
DURYSTA™ (bimatoprost intracameral implant) is indicated for the reduction of intraocular pressure (IOP) in patients with open angle glaucoma (OAG) or ocular hypertension (OHT).
An Overview of DURYSTA (bimatoprost intracameral implant)
DURYSTA (Allergan, an AbbVie company) is a first-in-class, biodegradable, intracameral implant for the lowering of intraocular pressure (IOP) in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT).1 DURYSTA was approved by the FDA in March 2020.2 The DURYSTA implant is inserted into the anterior chamber and comes to rest in the iridocorneal angle, where it provides a sustained release of bimatoprost for several months.1 Arsham Sheybani, MD; Francis Mah, MD; Randy Craven, MD; Jason Bacharach, MD; and Tosin Smith, MD, participated in the first of a 3-part series of roundtable discussions about DURYSTA. This first piece is focused on understanding the safety and efficacy of DURYSTA, as well as which patients might be best served by this novel treatment.
STUDY OUTCOMES
Arsham Sheybani, MD: The FDA approval of the first biodegradable, intracameral, sustained-release implant to reduce IOP in patients with OAG or OHT1 is a milestone in glaucoma management. Our goal here is to understand the efficacy and safety data and discuss how we will use this treatment with our patients. The phase 3 studies, ARTEMIS 1 and 2, were 2 identical, multicenter, randomized, parallel-group, controlled, 20-month studies, including an 8-month extended follow-up.1 In these studies, the efficacy of DURYSTA was compared to twice-daily topical timolol 0.5% drops in patients with OAG or OHT. DURYSTA is for single administration only and should not be re-administered to an eye that received a prior DURYSTA.1
When you look at the phase 3 data (Figure 1), the average baseline IOP was in the mid 20s, and treatment with DURYSTA provided up to 33% reduction (≈ 5-8 mm Hg) in pressure over the 12-week primary efficacy period. In your minds, which aspects of DURYSTA are most compelling to you, and how do you plan to use it within your practice?
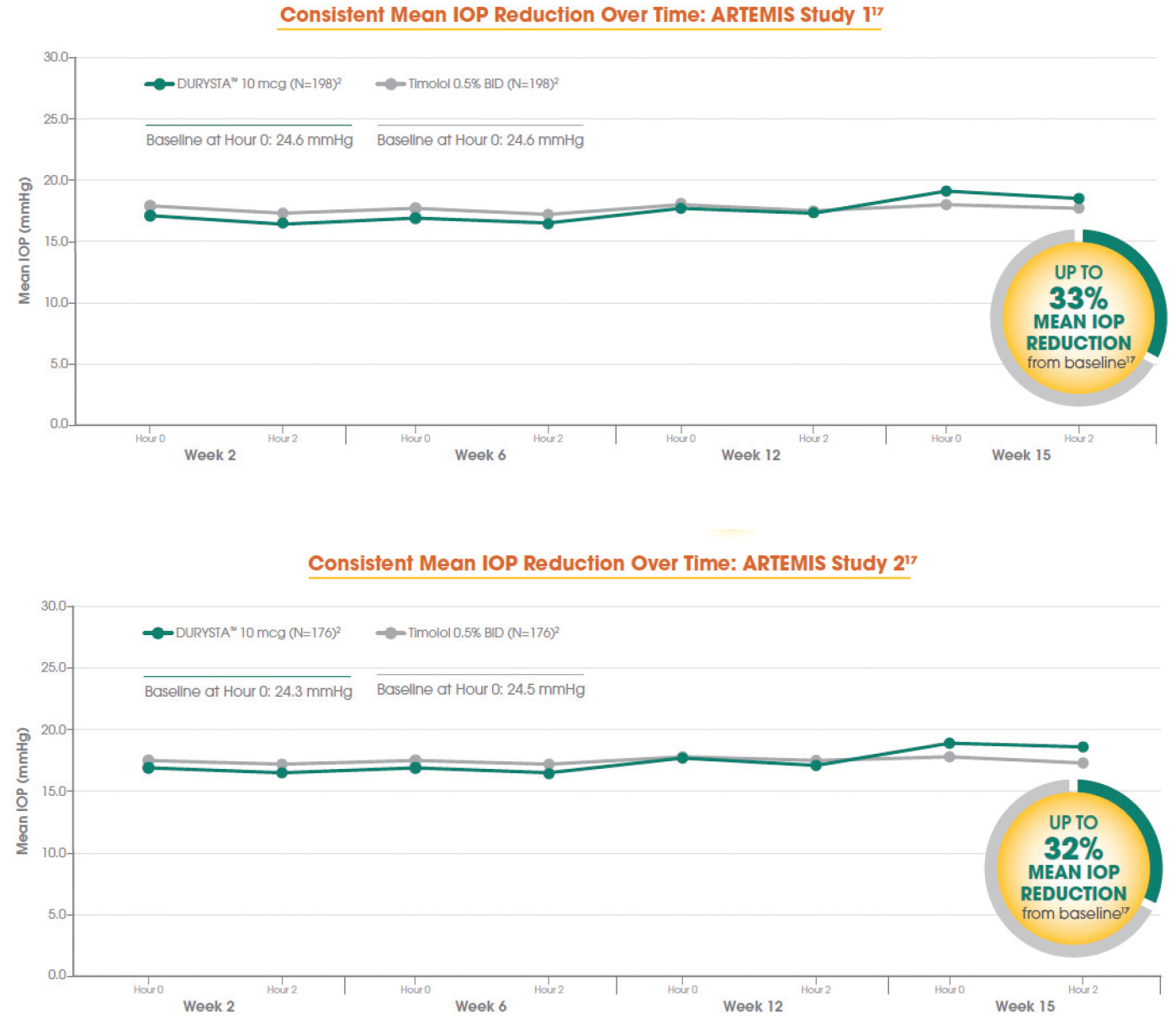
Figure 1. Data from the ARTEMIS 1 Trial (top) and the ARTEMIS 2 Trial (bottom).
Tosin Smith, MD: As clinicians, we’re looking at how many points of pressure reduction we need, and what can get us there. Looking at the numbers, up to 8 mm Hg of pressure reduction is effective in reducing eye pressure for my patients, and a single administration of DURYSTA provides us the ability to lower their IOP for several months.1
Jason Bacharach, MD: Dr. Sheybani and colleagues published data showing that a lot of IOP fluctuations occur outside of office hours and may not be detected.3 It will be interesting to see how our patients benefit from 24/7 delivery of medication for several months.1 I am looking forward to following my patients who’ve received DURYSTA to lower their IOP and monitoring their response to this sustained-release therapy.1
BENEFITS OF DURYSTA
Dr. Sheybani: I’d like to discuss some of the potential benefits of DURYSTA, in particular, physician-guided administration and targeted delivery to diseased tissues. The intracameral administration of DURYSTA allows the preservative-free implant to bypass the ocular surface and be placed within the anterior chamber angle, delivering drug to targeted tissues (Figure 2).1 What are the potential benefits of sustained-release therapies for lowering IOP in patients with primary OAG (POAG)?

Figure 2. The DURYSTA implant (red arrow) and applicator.
Dr. Smith: With DURYSTA, the clinician administers the medicine and knows that the patient is receiving therapy for several months without having to administer the medication on their own.1
Dr. Bacharach: There are some very important benefits to extended-release medications. In many other subspecialties of medicine, there are now options for sustained-release therapy. In ophthalmology, cataract surgeons can now inject sustained-release medicines postoperatively; in retina, we see sustained-release treatments being utilized regularly. The physician-guided administration of DURYSTA allows us to deliver medication in a way that bypasses the ocular surface, a physical barrier through which the medication must pass.1,4 In addition, with the current pandemic, while patients are still coming into my office for their regular follow-up visits, they are looking to make fewer trips outside of their home—specifically, fewer trips to the pharmacy.
Randy Craven, MD: Sustained-release drugs have many benefits. As Dr. Bacharach mentioned, there are benefits to different methods of delivering the drug to the tissue. The intracameral delivery of this sustained-release implant and its bypassing of the ocular surface allow for targeted delivery to the diseased tissues. Targeted delivery to the diseased tissues of the anterior chamber angle allows for just 10mcg of bimatoprost to provide sustained IOP control over the course of several months.1
Dr. Sheybani: From a cornea perspective, what is the clinical value of a sustained-release implant to lower IOP for your glaucoma patients?
Francis Mah, MD: As Dr. Bacharach mentioned, to reach the intended tissues, medication must permeate the hydrophilic and hydrophobic layers of the cornea.4 With DURYSTA, we have the ability to administer a medication that bypasses the ocular surface and put the medication directly within the anterior chamber angle.1
ADAPTING THE TREATMENT PARADIGM
Dr. Sheybani: Whenever you have a novel therapeutic modality, there needs to be discussion surrounding when and how to use it. Can you each summarize how you will use DURYSTA within your practice?
Dr. Bacharach: We’re really talking about putting a novel treatment into a paradigm that’s been around for a very long time. Minimally invasive glaucoma surgery (MIGS) treatments really opened up the treatment paradigm and found surgeons mixing and matching and getting excellent results. To start with, I wouldn’t pigeonhole it. The efficacy is good, and there are times when I may use DURYSTA prior to selective laser trabeculoplasty (SLT) and times I would use it afterward. Drop application can be challenging for patients with mental or physical comorbidities. I may consider DURYSTA early on in a patient who does not want drops, or for whom drops are not suitable (eg, inability to instill drops, forgetfulness). Bimatoprost is thought to lower IOP by increasing outflow of aqueous humor through both the trabecular meshwork (conventional) and uveoscleral (unconventional) pathways,1 making me very interested to see how it will work on my patients with whom I’ve used other interventions such as SLT. That is really intriguing to me as a physician.
Dr. Craven: Within my practice, DURYSTA is a sound treatment option for patients with mild to moderate glaucoma who need IOP lowering and require an alternative to topical therapy. That could be because they have difficulty remembering to take drops or applying drops. I think it is useful in pseudophakic patients as well. Initially, this is where I’ve found utility with this product. As I continue to gain experience with DURYSTA, I’ll likely move on to other indicated patient groups.
Dr. Smith: I would say that you can consider using DURYSTA before or after SLT, depending on what you are trying to achieve. Now I can offer DURYSTA or SLT to the patient who needs IOP lowering and either does not want or is not a good candidate for topical drops, as they may prefer the option to not need to take medication daily. We have patients with moderate disease and patients who are early in their treatment who are taking topical drops to lower their IOP but are looking for an alternative option. DURYSTA could be a great option for these patients. DURYSTA can also be an option for patients who could benefit from a preservative-free treatment, are between interventions, or are post SLT and have not yet received MIGS and need IOP lowering for their glaucoma.
Dr. Mah: From the perspective of a cornea specialist, an intracameral implant that does not sit on the ocular surface can be useful (Figure 3).5,6 I have always felt that options like MIGS and SLT should be more widely adopted, or at least considered along with drops, especially for patients who may be sensitive (or experience sensitivity) to preservatives.5,6 I would want people to be considering SLT, MIGS, and DURYSTA early in the treatment in addition to drops, where appropriate, and I think comprehensive ophthalmologists and cataract surgeons have also adopted this view. A significant percentage of glaucoma patients needing IOP lowering may experience some sensitivity to preservatives.7 I believe this should be considered when weighing treatment options.
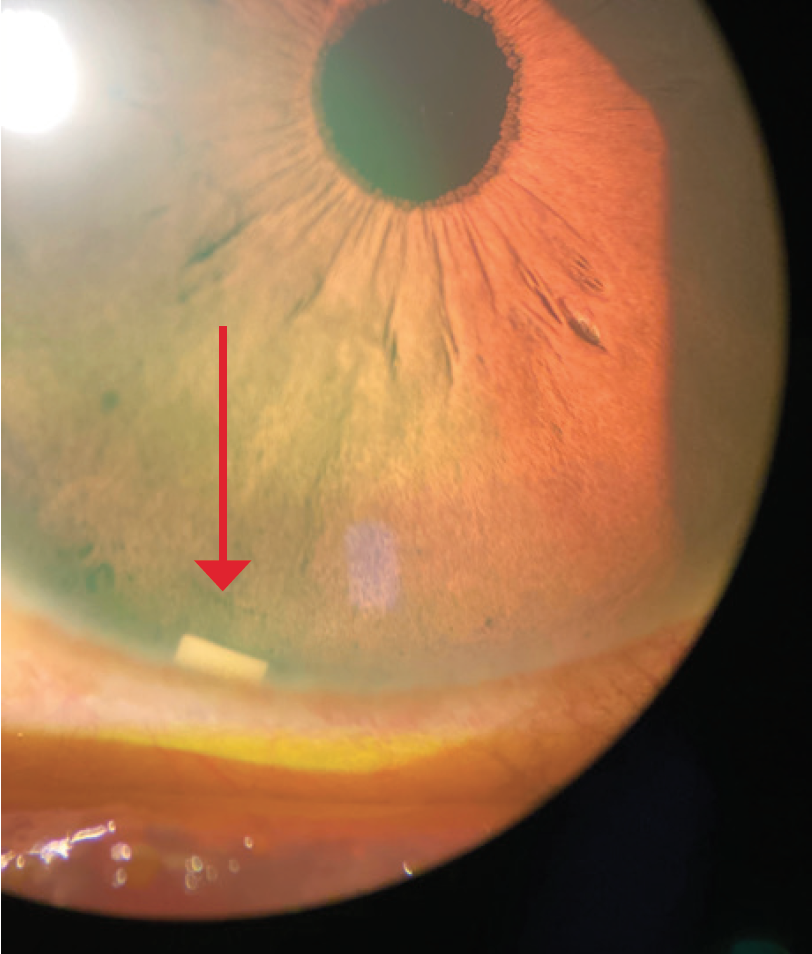
Figure 3. DURYSTA implant (red arrow).
Dr. Sheybani: In the phase 3 studies, following washout ranging 4 to 42 days depending on the treatment, a single administration of DURYSTA was able to control patients’ IOP for several months. What are the potential benefits of sustained IOP control?
Dr. Smith: More and more, I am consciously trying to monitor my patients’ treatment journey and make decisions on the appropriate time to take the next step. DURYSTA allows me to control a patient’s IOP for several months and gives me time before deciding which intervention to use next.
Dr. Mah: Several months of IOP control is an important consideration for patients who may be sensitive to preservatives. This will buy us time, as we consider what intervention comes next in the patient’s treatment process.
Dr. Sheybani: In the clinical trials for DURYSTA, the most common ocular adverse reaction was conjunctival hyperemia, which occurred in 27% of patients. Other common ocular adverse reactions reported in 5% to 10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, IOP increased, corneal endothelial cell loss, vision blurred, and iritis.1
Ocular adverse reactions occurring in 1% to 5% of patients were anterior chamber cell, lacrimation increased, corneal edema, aqueous humor leakage, iris adhesions, ocular discomfort, corneal touch, iris hyperpigmentation, anterior chamber flare, anterior chamber inflammation, and macular edema.1
The most common nonocular adverse event was headache, which was observed in 5% of patients.1
While there were no cases of endophthalmitis in the clinical trials,1 we know these types of intraocular, surgical procedures and injections have been associated with endophthalmitis, so proper aseptic technique must always be used when administering DURYSTA, and patients should be monitored following the administration.1 When you look at this, what stands out in your mind as truly significant and how will this impact your patient selection?
Dr. Smith: Patient selection will play a role in this procedure. Physicians will have to perform gonioscopy and make sure the angle is deep enough to receive the implant without touching the corneal endothelium.
Dr. Bacharach: As my colleagues have stated, you must look at the angle, and pick the right patients. DURYSTA should be used with caution in patients with narrow iridocorneal angles (Shaffer grade < 3) or anatomical obstruction (eg, scarring) that may prohibit settling in the inferior angle.1
THE PATIENT CONVERSATION
Dr. Sheybani: Whenever you have a novel treatment like this intracameral implant, you must also consider the patient conversation. We are the ones who first discuss this treatment option with our patients, so we need to be able to convey the risks and benefits and our treatment goals in a way that overcomes any natural reticence to injections. How do you talk to your patients about DURYSTA?
Dr. Craven: I think this is very easy to explain to patients. I tell them that we have an implantable medication to use, I explain why this is the option I’ve chosen for them, and I give the reasons why I recommend it. I explain that injections are frequently used in other types of ophthalmic conditions and are commonplace.
Dr. Bacharach: When I have a patient who is challenged by drop instillation and is looking for a different option, I tell them about DURYSTA. I explain that it is a sustained-release implant approved by the FDA to lower eye pressure in patients with OAG, and since I will be administering it, they don’t need to remember to take it every day.
Dr. Smith: I focus on discussing the benefits of the treatment. I explain that it is a different treatment that allows me to place their medication in the front part of their eye, where it is slowly released for several months, and without them having to administer their medication on their own. I explain that this is done in the office and why it may be beneficial to them, and with that, they are usually more receptive. If they ask specifically about the injection process, then I add more information. After the procedure, I counsel them that if their eye becomes red, sensitive to light, painful, or develops a change in vision, they should immediately call the office.1
Dr. Sheybani: DURYSTA represents a novel way to treat our patients—one that takes into account patients who are looking for a preservative-free option as well as those who may have difficulties administering medication on their own. This intracameral, biodegradable implant provides 24/7 drug delivery for several months targeted directly to the diseased tissue.1 Thank you for an excellent discussion on the outcomes of the clinical trials and how you are each translating that information into your individual practices.
DURYSTA™ Indications and Usage and Important Safety Information
Indications and Usage
DURYSTA™ (bimatoprost intracameral implant) is indicated for the reduction of intraocular pressure (IOP) in patients with open angle glaucoma (OAG) or ocular hypertension (OHT).
Important Safety Information
Contraindications
DURYSTA™ is contraindicated in patients with: active or suspected ocular or periocular infections; corneal endothelial cell dystrophy (e.g., Fuchs’ Dystrophy); prior corneal transplantation or endothelial cell transplants (e.g., Descemet’s Stripping Automated Endothelial Keratoplasty [DSAEK]); absent or ruptured posterior lens capsule, due to the risk of implant migration into the posterior segment; hypersensitivity to bimatoprost or to any other components of the product.
Warnings and Precautions
The presence of DURYSTA™ implants has been associated with corneal adverse reactions and increased risk of corneal endothelial cell loss. Administration of DURYSTA™ should be limited to a single implant per eye without retreatment. Caution should be used when prescribing DURYSTA™ in patients with limited corneal endothelial cell reserve.
DURYSTA™ should be used with caution in patients with narrow iridocorneal angles (Shaffer grade ˂ 3) or anatomical obstruction (e.g., scarring) that may prohibit settling in the inferior angle.
Macular edema, including cystoid macular edema, has been reported during treatment with ophthalmic bimatoprost, including DURYSTA™ intracameral implant. DURYSTA™ should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Prostaglandin analogs, including DURYSTA™, have been reported to cause intraocular inflammation. DURYSTA™ should be used with caution in patients with active intraocular inflammation (e.g., uveitis) because the inflammation may be exacerbated.
Ophthalmic bimatoprost, including DURYSTA™ intracameral implant, has been reported to cause changes to pigmented tissues, such as increased pigmentation of the iris. Pigmentation of the iris is likely to be permanent. Patients who receive treatment should be informed of the possibility of increased pigmentation. While treatment with DURYSTA™ can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly.
Intraocular surgical procedures and injections have been associated with endophthalmitis. Proper aseptic technique must always be used with administering DURYSTA™, and patients should be monitored following the administration.
Adverse Reactions
In controlled studies, the most common ocular adverse reaction reported by 27% of patients was conjunctival hyperemia. Other common adverse reactions reported in 5% 10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, intraocular pressure increased, corneal endothelial cell loss, vision blurred, iritis, and headache.
Please see full Prescribing Information.
© 2021 AbbVie. All rights reserved. DURYSTA™ and its design are trademarks of Allergan, Inc., an AbbVie company. DurystaHCP.com DUR143510 03/21
1. DURYSTA™ Prescribing Information.
2. US Department of Health and Human Services. Supplemental approval. NDA 211911. Drugs @ FDA website. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2020/211911Orig1s000ltr.pdf. Published March 4, 2020. Accessed September 14, 2020.
3. Sheybani A, Scott R, Samuelson TW, et al. Open-angle glaucoma: Burden of illness, current therapies, and the management of nocturnal IOP variation. Ophthalmol Ther. 2020;9(1):1-14.
4. Bachu RD, Chowdhury P, Al-Saedi ZHF, et al. Ocular drug delivery barriers-role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics. 2018;10(1):28.
5. Zhang X, Vadoothker S, Munir WM, et al. Ocular surface disease and glaucoma medications: A clinical approach. Eye Contact Lens. 2019;45(1):11-18.
6. Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86(4):418-423.
7. Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350-355.
DURYSTA™ Important Safety Information for US Healthcare Professionals
Collapse -
Contraindications
DURYSTA™ is contraindicated in patients with: active or suspected ocular or periocular infections; corneal endothelial cell dystrophy (e.g., Fuchs’ Dystrophy); prior corneal transplantation or endothelial cell transplants (e.g., Descemet’s Stripping Automated Endothelial Keratoplasty [DSAEK]); absent or ruptured posterior lens capsule, due to the risk of implant migration into the posterior segment; hypersensitivity to bimatoprost or to any other components of the product.
Warnings and Precautions
The presence of DURYSTA™ implants has been associated with corneal adverse reactions and increased risk of corneal endothelial cell loss. Administration of DURYSTA™ should be limited to a single implant per eye without retreatment. Caution should be used when prescribing DURYSTA™ in patients with limited corneal endothelial cell reserve.
DURYSTA™ should be used with caution in patients with narrow iridocorneal angles (Shaffer grade ˂ 3) or anatomical obstruction (e.g., scarring) that may prohibit settling in the inferior angle.
Macular edema, including cystoid macular edema, has been reported during treatment with ophthalmic bimatoprost, including DURYSTA™ intracameral implant. DURYSTA™ should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Prostaglandin analogs, including DURYSTA™, have been reported to cause intraocular inflammation. DURYSTA™ should be used with caution in patients with active intraocular inflammation (e.g., uveitis) because the inflammation may be exacerbated.
Ophthalmic bimatoprost, including DURYSTA™ intracameral implant, has been reported to cause changes to pigmented tissues, such as increased pigmentation of the iris. Pigmentation of the iris is likely to be permanent. Patients who receive treatment should be informed of the possibility of increased pigmentation. While treatment with DURYSTA™ can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly.
Intraocular surgical procedures and injections have been associated with endophthalmitis. Proper aseptic technique must always be used with administering DURYSTA™, and patients should be monitored following the administration.
Adverse Reactions
In controlled studies, the most common ocular adverse reaction reported by 27% of patients was conjunctival hyperemia. Other common adverse reactions reported in 5% 10% of patients were foreign body sensation, eye pain, photophobia, conjunctival hemorrhage, dry eye, eye irritation, intraocular pressure increased, corneal endothelial cell loss, vision blurred, iritis, and headache.
Please see full Prescribing Information.








