CASE PRESENTATION
A 72-year-old man from Ethiopia presents for a cataract evaluation. The patient states that the vision in his right eye has always been better than the vision in his left eye but that the latter has decreased dramatically during the past 2 years.
On examination, the patient’s UCVA is 20/40 OD and counting fingers at 2 feet OS that does not improve with refraction. Mild corneal scarring and a 2+ nuclear sclerotic cataract with 2+ cortical changes are evident in the right eye. Inferonasal corneal scarring and a dense white cataract are present in the left eye (Figures 1 and 2). A dilated funduscopic examination and B-scan ultrasonography of the posterior segment of the left eye are unremarkable.
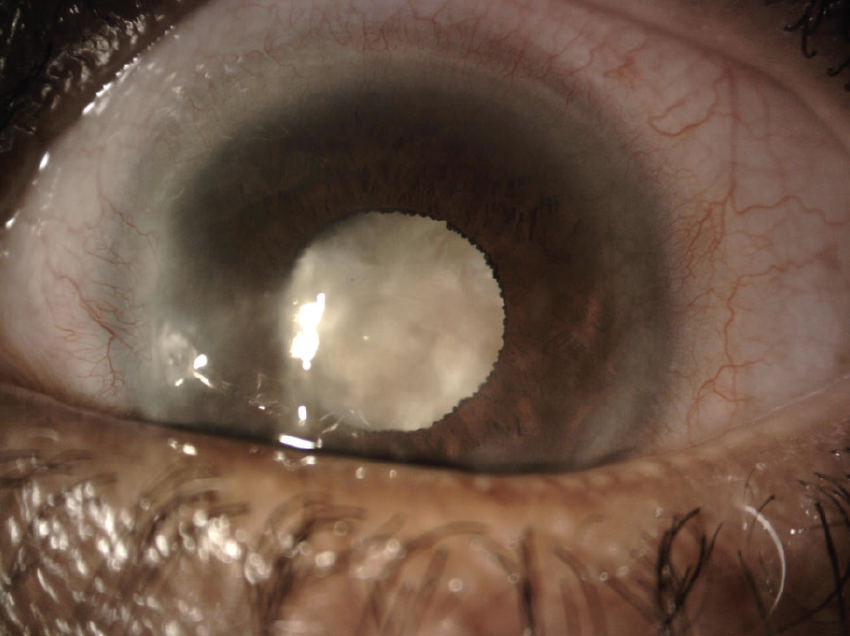
Figure 1. Dense cataract and corneal scarring in the left eye.
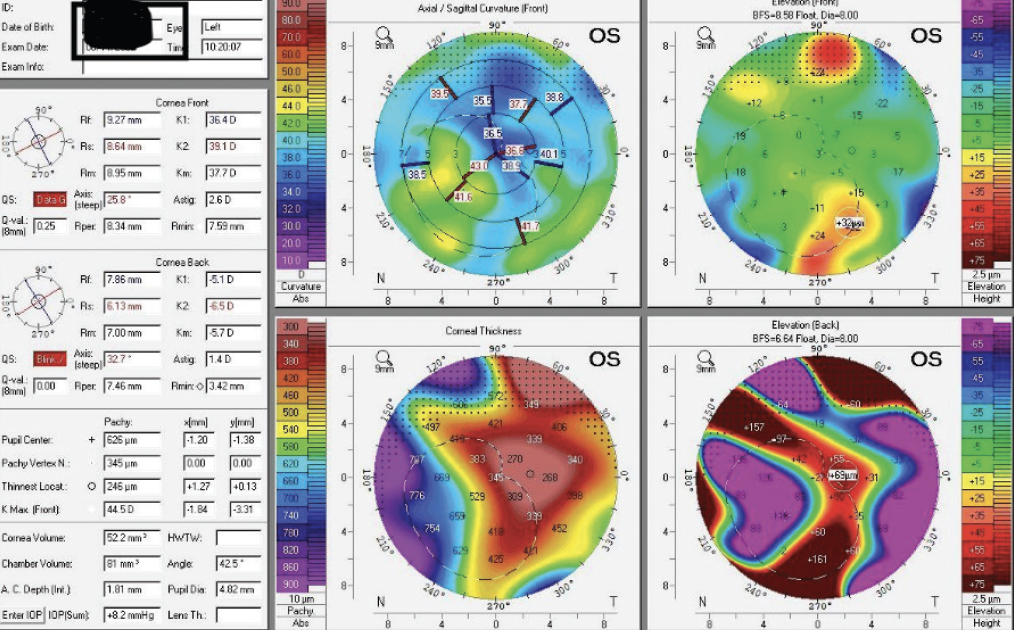
Figure 2. Tomography with the Pentacam shows irregular astigmatism in the left eye.
Would you perform cataract surgery alone or a corneal procedure first? When planning cataract surgery, which keratometry (K) readings would you use for the IOL calculations, and which IOL(s) would you consider? How would you approach surgery?
—Case prepared by Audrey R. Talley Rostov, MD



JESSICA HECKMAN, OD; BLAKE HAMPTON, MD; AND Y. RALPH CHU, MD
Given the dense white cataract, we recommend performing cataract surgery first. Despite the presence of corneal pathology, the cataract is likely the main contributor to the patient’s decreased visual acuity. Corneal surgery can be considered as necessary once the full visual effect of the opacity is determined after cataract removal.
Using multiple methods of keratometry could help provide a more complete evaluation of the cornea. We would favor K values obtained with Scheimpflug imaging for the IOL calculation, but Placido-based topography and manual keratometry could provide additional insight. Secondary to the irregular astigmatism induced by corneal pathology, IOL options include an aspheric monofocal lens, the Light Adjustable Lens (RxSight), and an IC-8 Apthera (AcuFocus), which was recently approved by the FDA. Given the patient’s potential need for future corneal surgical intervention or a specialty contact lens, an aspheric monofocal IOL would be our preference.
Manual small-incision cataract surgery, phacoemulsification, and laser cataract surgery are all options. The density of the cataract increases the risk of trauma during phacoemulsification, and corneal scarring could interfere with the beam of a femtosecond laser. Manual small-incision cataract surgery would therefore be our preference. A stretching technique or an expansion device would be used to address the poorly dilated pupil. The anterior capsule would be stained with trypan blue dye to aid visualization during capsulorhexis creation in the absence of a red reflex.

SOMASHEILA I. MURTHY, MD
Preoperatively, I would counsel the patient on realistic expectations for his visual results. Cataract surgery would be undertaken first because the lens exhibits an intumescent advanced cataract and a delay may lead to lens-induced uveitis.
A peribulbar block would work well for akinesia and a soft globe. In patients of this age, an Argentinian flag–like extension is less likely than in younger patients, but controlling the centration and size of the capsulorhexis can be challenging. My preferred technique is to stain the anterior capsule with trypan blue dye (Auroblu, Aurolab). Under a cohesive OVD, a nick would be made with a bent 26-gauge needle, and the loose cortical material would be aspirated from the center of the capsular bag. After replenishing the OVD, the nick would be enlarged to create an arrowhead flap, and the capsulorhexis would be completed with repeated maneuvers of the needle mounted on a syringe filled with a dispersive OVD. I would quickly switch to microrhexis forceps if required. The use of a femtosecond laser or Zepto Capsulotomy System (Centricity Vision) could also be considered, but I feel confident that a manual capsulorhexis can be achieved in this case.
The IOLMaster 700 (Carl Zeiss Meditec) would be used for biometry, and the Barrett Universal II formula would be used for the IOL calculation. If a large discrepancy between these measurements and corneal tomography is found, values obtained with the Pentacam (Oculus Optikgeräte) would be entered into the Barrett True-K formula.
A one-piece hydrophobic nonaspheric IOL would be implanted. I would avoid a toric IOL because of the irregular astigmatism.

PRIYANKA SOOD, MD
It may be possible to improve the patient’s quality of vision dramatically.
A short course of steroids and aggressive lubrication of the ocular surface would be initiated in case ocular surface disease is present. Once the topography is stable, my preference would be to perform cataract surgery first because corneal scars, especially when located outside the visual axis, are sometimes less visually significant than they appear on examination.
Preoperatively, it would be crucial to set realistic expectations and to develop a clear understanding of the patient’s needs and his ability to optimize his vision over the long term. If he can wear a rigid gas permeable or scleral lens, there is some wiggle room on the refractive outcome of surgery.
When performing the IOL calculations, I would focus on the central 4.5-mm Holladay equivalent K values on the Pentacam. The average would be compared to the biometry measurements and K readings. If there is a large discrepancy in the average K values, I would err toward the central 4-mm ring measurement and enter it into the formula. An aspheric, aberration-free monofocal IOL such as the enVista (Bausch + Lomb) would be my preference because it would not increase higher-order aberrations.
Pupillary dilation appears to be necessary for surgery. I prefer to use iris hooks in a situation like this because they can be implanted without the use of an OVD, which allows the staining of the capsule with trypan blue dye to be darker. I also find hooks to be more amenable to the use of a miLoop (Carl Zeiss Meditec) than intraocular pupillary dilation devices, which can become entangled with the miLoop during nuclear division.
I would expect the patient to be happy with the surgical outcome if realistic expectations are set preoperatively.


CARA G. CAMPBELL, BMEDSC, BMBS, MSC, AND ANDREW M.J. TURNBULL, BM, PGCERTMEDED, PGDIPCRS, CERTLRS, FRCOPHTH
The history suggests that the patient’s left eye is amblyopic. It would be useful to know the likely visual potential from previous optometric records. The possibility of prior ocular trauma and subsequent zonulopathy should also be considered.
Optimizing the ocular surface and tear film, with or without a superficial keratectomy, may facilitate more accurate keratometry. The central cornea is relatively clear. The scarring is inferonasal and likely predates cataract onset, so we would defer corneal surgery until after cataract surgery and after more is known about the patient’s visual potential.
A toric IOL is likely an inappropriate choice, but the K calculator integrated into the Barrett Toric Calculator can be used to generate a mean or median vector K reading from two or three devices to improve reliability. A modern high-performance IOL calculation method (eg, Barrett Universal II, Kane, Hill-RBF 3.0) would be used primarily, but the Haigis formula could be used for comparison because it is less influenced by inaccurate K readings. Regardless, the patient would be counselled on the likelihood of a residual refractive error.
Cataract surgery alone with the implantation of a standard monofocal IOL should produce a rapid and significant improvement in vision. Afterward, the impact of corneal scarring and the potential benefit of further rehabilitation can be determined. Any residual refractive error can be managed with a piggyback IOL. Alternatively, topography-guided PRK could be used to treat residual ametropia and regularize the corneal surface.
An alternative worth considering is the implantation of the IC-8 Apthera because it can reduce peripheral aberrations and improve quality of vision in eyes with irregular astigmatism.
In addition to the use of trypan blue dye during cataract surgery, intravenous mannitol, tamponade with Healon5 (Johnson & Johnson Vision), and needle decompression would be performed to reduce the risk of a run-out capsulorhexis. Iris retractors would be available for use to improve pupillary dilation and support the capsule if zonulopathy is encountered. A three-piece IOL, capsular tension segments, a capsular tension ring, and anterior vitrectomy equipment would also be on standby.

WHAT I DID: AUDREY R. TALLEY ROSTOV, MD
IOL calculations were performed with the IOLMaster 700 using the Barrett Universal and True-K formulas. K readings obtained with the Pentacam, Cassini (i-Optics), and IOLMaster were all slightly different, so I selected a nontoric three-piece IOL that could be placed in either the bag or the sulcus.
A peribulbar block was administered. A laser capsulotomy was created with the Lensar Laser System (Lensar) and centered on the capsular bag. Imaging during this step suggested an area of zonulopathy.
The capsule was stained with trypan blue dye, a dispersive OVD was instilled, and the capsulotomy was removed without complications. Bimanual phacoemulsification was performed, and a cohesive OVD (Provisc, Alcon) was injected, separating the cortex from the capsule with viscodissection.
A capsular tension ring was placed before cortical cleanup was performed. After bimanual irrigation and aspiration, a Sofport AO IOL (model LI61AO, Bausch + Lomb) was placed in the capsular bag.
The patient did well after surgery. His BCVA was 20/50, and he was thrilled with his result.




