
We cataract surgeons constantly strive to improve the refractive accuracy of our surgeries. Over the decades, we have refined our IOL power calculation formulas and invested in increasingly sophisticated biometric measurement devices in our quest for precision. Despite our endeavors, however, the factor that derails our best efforts in many cases is a patient’s unhealthy ocular surface. Untreated dry eye disease (DED) is the elephant in the room in regard to achieving excellent refractive results in cataract surgery.
My colleagues, Alice T. Epitropoulos, MD, FACS; Eric D. Donnenfeld, MD; and Jack T. Holladay, MD, MSEE, FACS; and I undertook a study to determine whether proactive treatment of the ocular surface—even in unsymptomatic patients—could improve refractive prediction in cataract surgery. The results of the study surprised us, demonstrating that a preoperative course of a topical DED treatment led to significant improvements not only in biometric accuracy but also in several other measures of surgical success.
We presented the results of this study at AAO 2019,1 and we intend to publish our full results in the peer-reviewed literature, but we thought, in the meantime, it would be of interest to CRST readers to learn about some of our topline results, which are summarized in this article.
STUDY DESIGN
The study was designed to evaluate how a preoperative 4-week course of topical treatment with lifitegrast ophthalmic solution 5% (Xiidra, Novartis) would affect the accuracy of preoperative biometry in predicting postoperative spherical equivalent (SE). To do this, we enrolled patients with cataract and significant DED—as indicated by decreased tear breakup time (TBUT) and increased corneal staining—and measured their biometry both before and after 4 weeks of treatment with lifitegrast.
Baseline measurements, in addition to SE, included root mean square (RMS) higher-order aberrations (HOAs) in the central 6 mm of the cornea, patients’ scores on the Standardized Patient Evaluation of Eye Dryness (SPEED) questionnaire, corneal staining, conjunctival redness, and TBUT. These measurements were repeated after 4 weeks of lifitegrast treatment.
IOL selection for surgery was based on whichever biometry (before or after lifitegrast) the surgeon chose. Patients’ postoperative refractive outcomes were measured at 1 month postoperatively, their SE refractive error was determined, and this was compared to the SE predicted by each of the preoperative biometry measurements (before and after lifitegrast). The absolute prediction error was compared between the two sets of biometric measurements, along with the RMS HOA in the central 6 mm of the cornea. The other measurements taken at baseline were also repeated.
Patients then completed surgery, finished their postoperative drops, and undertook another 4 weeks of twice-daily lifitegrast treatment. The same outcomes were measured once again, about 2 months after surgery. This step was done to investigate whether patients with DED benefited from continued treatment with lifitegrast after their cataract surgery treatment course was completed.
RESULTS
We found that biometry performed after lifitegrast treatment predicted patients’ refractive outcomes significantly more accurately—that is, with significantly less absolute prediction error—than biometry performed before lifitegrast treatment (P < .04, paired t-test; Figure 1).
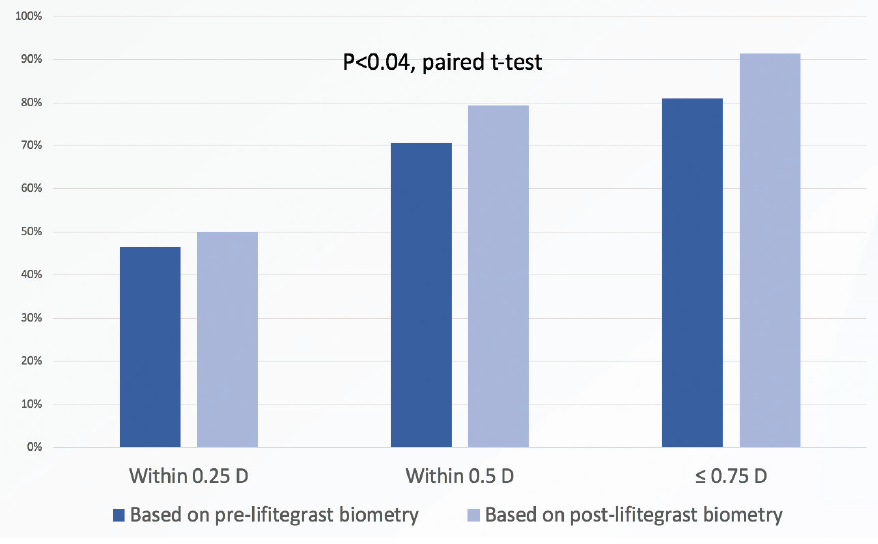
Figure 1. Biometry performed after lifitegrast treatment predicted the postoperative refractive outcome significantly more accurately than when biometry was performed before lifitegrast (paired t-test).
Furthermore, lifitegrast treatment for 1 month before surgery led to statistically significant improvement in HOAs (P < .001, paired t-test), and that benefit persisted at the final visit in stable, pseudophakic patients (P < .004). After 1 month of preoperative treatment with lifitegrast, HOAs improved by a mean 0.16 ±0.33 µm in 56% of patients and, by the final visit, HOAs had improved by 0.37 ±0.29 µm in 54% of patients.
The improvement that was noted in HOAs suggests that significantly more lifitegrast-treated than nontreated patients are likely to be satisfied with their visual quality after refractive cataract surgery (Figure 2). That is, patients are more likely to be satisfied with their vision with multifocal IOLs if their RMS HOAs are less than 0.5 µm.2,3 In this study, 32% of patients had less than 0.5 µm of RMS HOAs before lifitegrast treatment, and 52% achieved that level after 4 weeks of lifitegrast treatment.
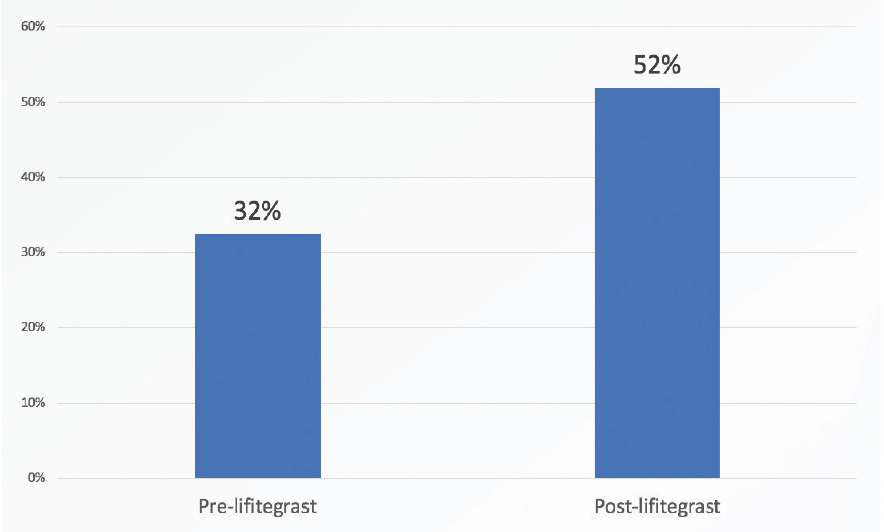
Figure 2. Significantly more lifitegrast-treated versus nontreated patients were satisfied with their visual quality after refractive cataract surgery.
Lifitegrast treatment improved signs of DED, including corneal staining, conjunctival redness, and TBUT. It also improved symptoms of DED, as indicated by improved scores on the SPEED test.
DISCUSSION
The results of this study surprised us in several ways. Most of us think of drugs such as lifitegrast as being too slow-acting to be beneficial in preparing patients for cataract surgery. This study, however, corroborates the FDA data on lifitegrast, which showed improvement in DED signs and symptoms after as little as 2 weeks of treatment.4 Additionally, it’s a product they can stay on for life to maintain the good vision they achieve after surgery. This was another important take-home point from this study: Patients should continue on DED treatment in order to enjoy sustained visual benefit. We can do the world’s best surgery, but, if we don’t continue to treat dry eye, we end up leaving the patient with suboptimal vision.
Originally, when Drs. Donnenfeld, Epitropoulos, Holladay, and I put this study together, we planned to recruit 200 patients because we thought it would be difficult to demonstrate a difference in refractive accuracy with fewer patients. However, after about 50 patients, we found that we had already reached statistical significance on a number of outcome parameters, and we truncated the study to include only 100 patients. Even at this sample size, we found statistical significance on refractive accuracy and all other outcome measures.
My colleagues and I believe that our study is a valuable follow-on to the Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study by Trattler et al.5 That study found that most patients presenting for cataract surgery had some signs of DED. Our study showed definitively that proactive treatment of these patients can make a world of difference in their refractive outcomes.
CONCLUSION
The message, of course, is not only about lifitegrast but rather about employing whatever methods are needed to control patients’ DED before they undergo the biometry that will determine the IOL power we select for surgery. We cannot emphasize this point strongly enough in teaching our colleagues.
1. Hovanesian JA, Epitropoulos A, Donnenfeld ED. The effect of lifitegrast on refractive accuracy, higher order aberrations, and symptoms in dry eye patients undergoing cataract surgery. Paper presented at: AAO 2019; October 12-15, 2019.
2. McCormick GJ, Porter J, Cox IG, MacRae S. Higher-order aberrations in eyes with irregular corneas after laser refractive surgery. Ophthalmology. 2005;112(10):1699-709.
3. Mirzajani A, Aghataheri S, Ghoreishi M, Jafarzadepour E, Mohammadinia M. Evaluation of corneal higher order aberrations in normal topographic patterns. J Curr Ophthalmol. 2016;28(2):75-80.
4. Xiidra [package insert]. Lexington, MA: Shire; 2017.
5. Trattler WB, Majmudar PA, Donnenfeld ED, McDonald MB, Stonecipher KG, Goldberg DF. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol. 2017;11:1423-1430.




