
Dry eye disease (DED) is one of the most common conditions seen by eye care physicians (ECPs). For many, it is also the bane of their existence. It is estimated that the overall prevalence of DED is approximately 14.5%; it is 17.9% among women and 10.5% among men.1 DED is also known as dysfunctional tear syndrome (DTS), among other names, as Cynthia Matossian, MD, FACS, outlines in the opening article. And, although managing these patients may be the least appealing part of the ECP practice, one could argue that it is the most important to address.
Our knowledge of DED has evolved over the past 20 years. We are now more certain that DED is an inflammatory process that may progress if left untreated. We also have a better understanding of the importance of the tear film, the first interface that light hits as it enters the eye, for vision. And we understand that failing to treat DED affects not only vision but also IOL calculations and surgical outcomes (see the article by Ashley Brissette, MD, MSc, FRCSC). Additionally, DED affects every specialty of the eye care practice.
So why do many ECPs often dread seeing a DED patient on their schedule? Perhaps because DED is a complex disease and often difficult to diagnose and treat. DED has multiple risk factors and triggers, varying and disparate signs and symptoms, and differing presentations. Further, patients with DED are frustrated, they often seek second opinions, and their care can be time-consuming, as treatment options may not always be apparent or fully effective.
One treatment option that can help in these situations is hormone therapy. It is often not the one that we reach for as a first-line treatment option; however, in some patients it can be highly effective.
This article answers five questions you should know about the use of topical hormone therapy in the treatment of DED.
No. 1: What is hormone therapy, and what are our options? Sex hormones regulate lacrimal gland and meibomian gland secretions. They not only have effects on protein synthesis and secretion; meibum, mucous, and aqueous output; and tear film stability; but also on epithelial cell health and immune activity.2,3 Hormonal supplements have been studied in DED, and they may provide benefit by improving secretion and suppressing inflammation.4,5
Topical hormones are compounded as drops. Some available options include medroxyprogesterone acetate 1%, progesterone 0.5%/testosterone 0.5% combination, and dehydroepiandrosterone (DHEA) 0.5–1% (topical androgen).6
Topical medroxyprogesterone, which may have anticollagenolytic activity, has been used for other ocular surface problems, such as for the treatment of alkali burns. It’s use is associated with a reduction in corneal perforation and ulceration and the healing of persistent epithelial defects.6,7
Androgens have antiinflammatory effects. Zibandeh et al showed that cultured corneal epithelial cells treated with dihidrotestosterone inhibited the proinflammatory cytokines tumor necrosis factor-alpha, interleukin (IL)-8, and IL-6.2
No. 2: Why even consider hormone therapy? The answer is simple: Sometimes treatment requires thinking outside the box. One reason many clinicians despise treating DED is that, although options are improving, treatments are still limited in number. Two formulations of topical cyclosporine, ophthalmic emulsion 0.05% (Restasis, Allergan) and ophthalmic solution 0.09% (Cequa, Sun Ophthalmics), and lifitegrast ophthalmic solution 5% (Xiidra, Novartis) are the commercially available treatments, and each is intended to treat the heart of the problem of DED by targeting inflammation.
In my opinion, they all work well—but one of the biggest mistakes in treating DED is the assumption that these treatments are a panacea. Like glaucoma, DED is a disease that often needs multiple treatments. A person with more than mild DED may need two or three treatment options to effect improvement. When a patient needs something additive to cyclosporine or lifitegrast, topical hormones as compounded drops may be an effective adjunctive option.
No. 3: What are the advantages of hormone therapy? Hormones are important in ocular surface homeostasis. Compounded topical hormonal treatments have been used anecdotally to treat DED with success.6 They are easy to use and are dosed as one drop given two to six times daily. There are minimal side effects and no known reported systemic adverse events.
Hormones have antiinflammatory effects and may improve both the signs and symptoms of DED. Topical compounded medications, such as topical hormones, may be an excellent option when a clinician needs more than what is commercially available.
No. 4: What are the disadvantages of hormone therapy? There is limited research and clinical evidence to definitively prove the benefits of topical hormones for the treatment of DED. It is important for ECPs to understand that many of these preparations, although apparently safe and with anecdotal success reported, have not been fully investigated for efficacy and safety. Therefore, their use is to be left to the judgment of each clinician.
No. 5: Where does hormone therapy fit into DED algorithms? Algorithms for DED, such as those from the Dysfunctional Tear Syndrome International Task Force (ITF),8 the Tear Film and Ocular Surface Society’s Dry Eye Workshop II (DEWS II),9 and the CEDARS DTS group,6 all provide roadmaps to the treatment of DED in an attempt to simplify the approach to this complex disease.
The first two, the ITF guidelines and the DEWS II, are severity-based algorithms, meaning that their treatment approach is based on the severity of the patient’s DED. In these algorithms, topical hormones would be considered in more severe cases.
The CEDARS algorithm, by contrast, uses a diagnosis-based approach, directing treatment based on four subcategories of DED: aqueous deficiency; evaporative DED, based on goblet cell deficiency; blepharitis with and without evaporative tear syndrome; and exposure-related DED (Table). Given the potential antiinflammatory effects of topical hormones, CEDARS lists them as a possible therapeutic option in aqueous-deficient DED, but they may also have benefit in evaporative DED based on goblet cell deficiency.
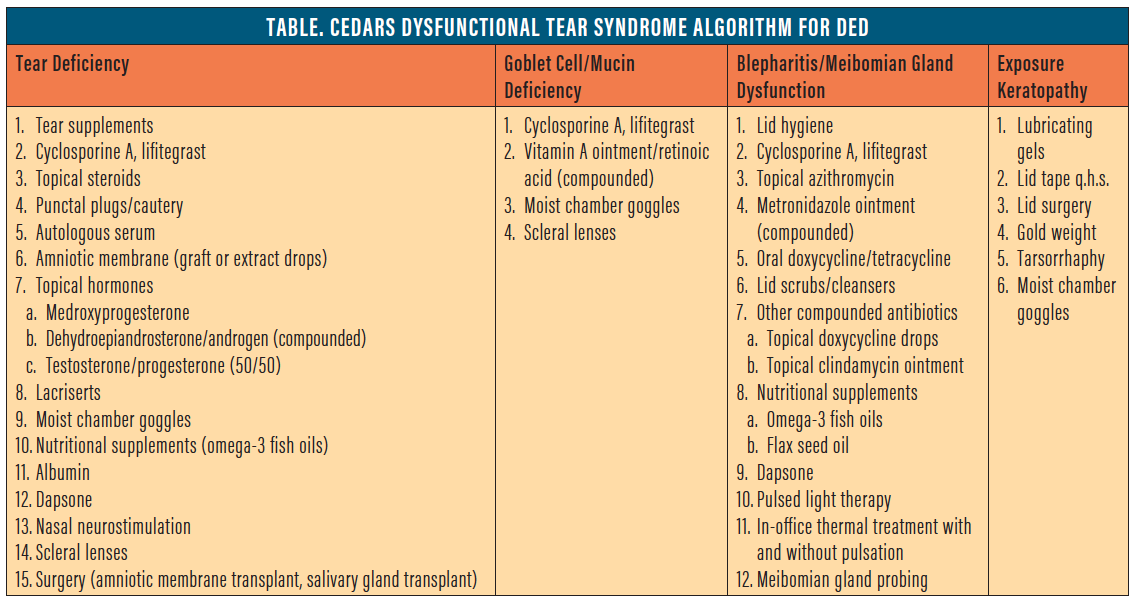
Basic research shows that androgens are involved in stimulating the process of lipidogenesis in the meibomian glands.6,10 Topical DHEA, therefore, may be useful in treating DED in the blepharitis/meibomian gland dysfunction category. Anecdotal reports and a case study review have discussed potential improvement in signs and symptoms in patients treated with DHEA for posterior blepharitis.5,6 However, there are no studies to evaluate its efficacy.
CONCLUSION
DED can often be successfully treated with the commercially available agents cyclosporine and lifitegrast, along with the standard regimen of warm compresses, artificial tears, and punctal occlusion. However, patients with DED often need additional therapies to achieve clinical success in alleviation of signs and symptoms. Topical compounded hormones may be an excellent option when the ECP needs more than what is commercially available.
1. Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157(4):799-806.
2. Zibandeh N, Yıldız E, Özer B, Taş AY, Sahin A. Androgen suppresses hyperosmolarity-induced inflammatory mediators in human corneal epithelial cells. Cornea. 2020;39(7):886-891.
3. Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938-1978.
4. Zhang X, M VJ, Qu Y, et al. Dry eye management: targeting the ocular surface microenvironment. Int J Mol Sci. 2017;18(7):1398.
5. Worda C, Nepp J, Huber JC, Sator MO. Treatment of keratoconjunctivitis sicca with topical androgen. Maturitas. 2001;37(3):209-212.
6. Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders – new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27 Suppl 1(Suppl 1):3-47.
7. Newsome NA, Gross J. Prevention by medroxyprogesterone of perforation in the alkali-burned rabbit cornea: inhibition of collagenolytic activity. Invest Ophthalmol Vis Sci. 1977;16(1):21-31.
8. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25(8):900-907.
9. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628.
10. Schirra F, Richards SM, Liu M, Suzuki T, Yamagami H, Sullivan DA. Androgen regulation of lipogenic pathways in the mouse meibomian gland. Exp Eye Res. 2006;83(2):291-296.




