CASE PRESENTATION
A 72-year-old woman recently underwent cataract surgery on her left eye. The patient states that she is eager to undergo cataract surgery on her right eye but “not until my left eye is fixed.”
In the early 1990s, she underwent eight-cut radial keratotomy (RK) with astigmatic keratotomy in each eye. The surgeon used the Lindstrom mini-RK technique and subsequently performed multiple enhancements. The patient developed progressive hyperopia and refractive instability.
In 1999, she was referred to me. I performed the lasso procedure developed by R. Bruce Grene, MD, to address the overly flat cornea. Several years later, the lasso suture broke and was removed, after which regression and central corneal flattening recurred. The patient regularly visited a local eye care provider for nearly 4 years, at which time she was referred to a glaucoma specialist. The patient was diagnosed with normal-tension glaucoma, moderate in the right eye and severe in the left eye. Progressive nuclear sclerotic cataracts were also evident.
In August 2019, the patient underwent cataract surgery and placement of an Ex-Press Glaucoma Filtration Device (P-50, Alcon) with a 1-minute application of mitomycin C in her left eye. Based on 3.5-year-old measurements with the Lenstar (Haag-Streit; Figures 1 and 2) and Galilei G4 (Ziemer Ophthalmic Systems; Figures 3 and 4) and intraoperative measurements with the ORA System (Alcon), a 22.50 D AcrySof IQ monofocal IOL (SN60WF, Alcon) was inserted into the capsular bag.
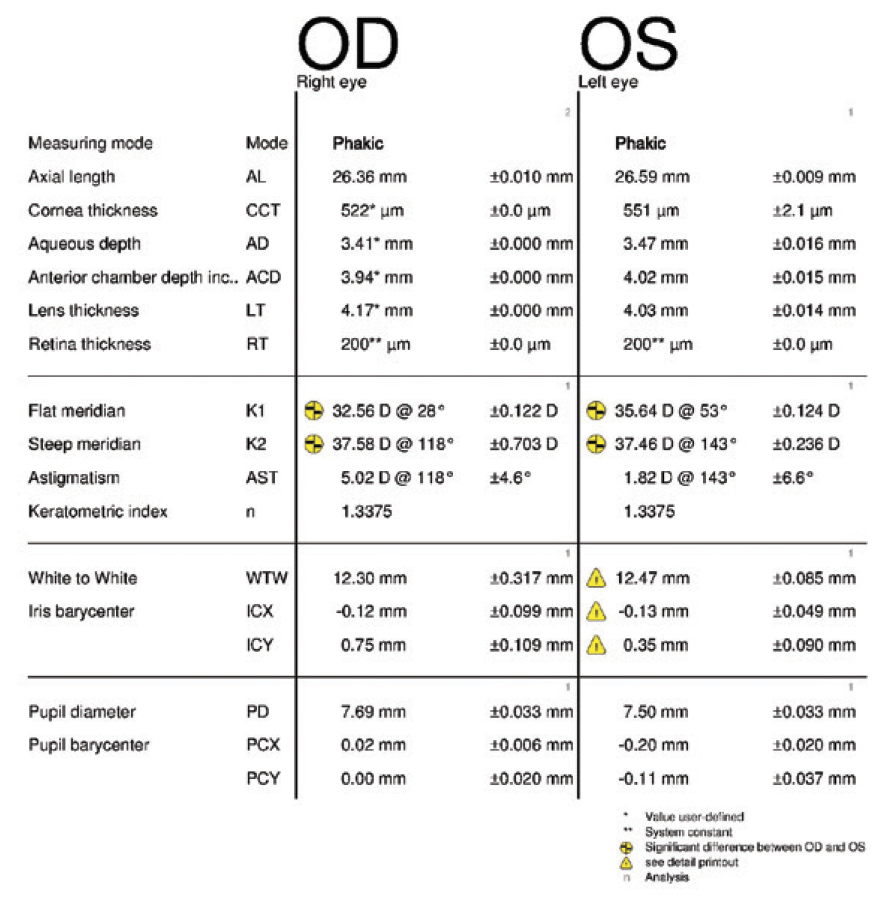
Figure 1. Measurements obtained 3.5 years before cataract surgery on the left eye.
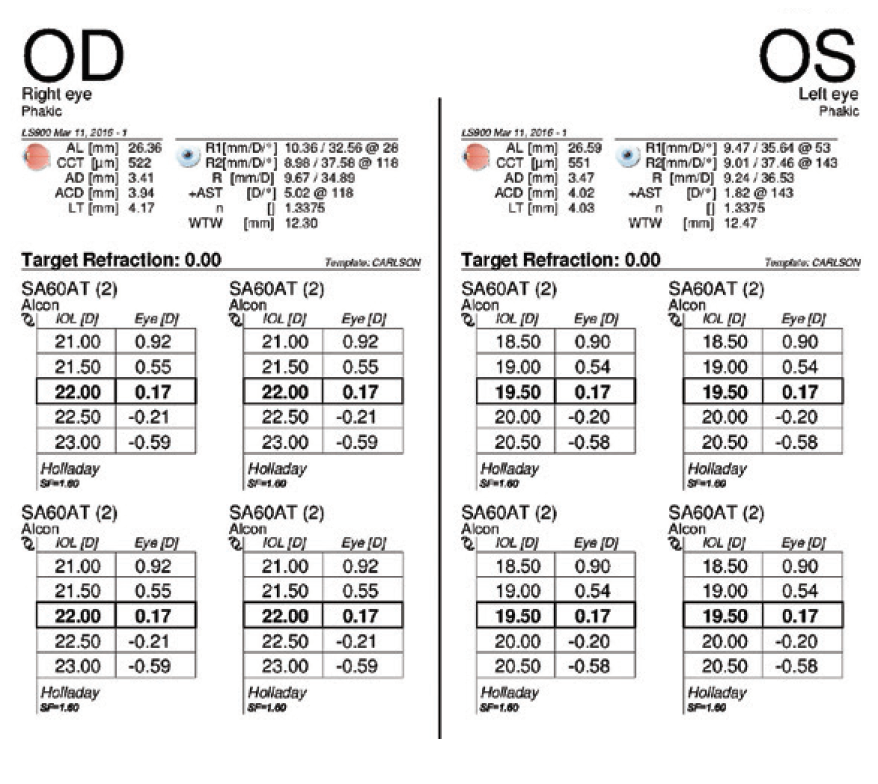
Figure 2. IOL power calculations performed 3.5 years before cataract surgery on the left eye.
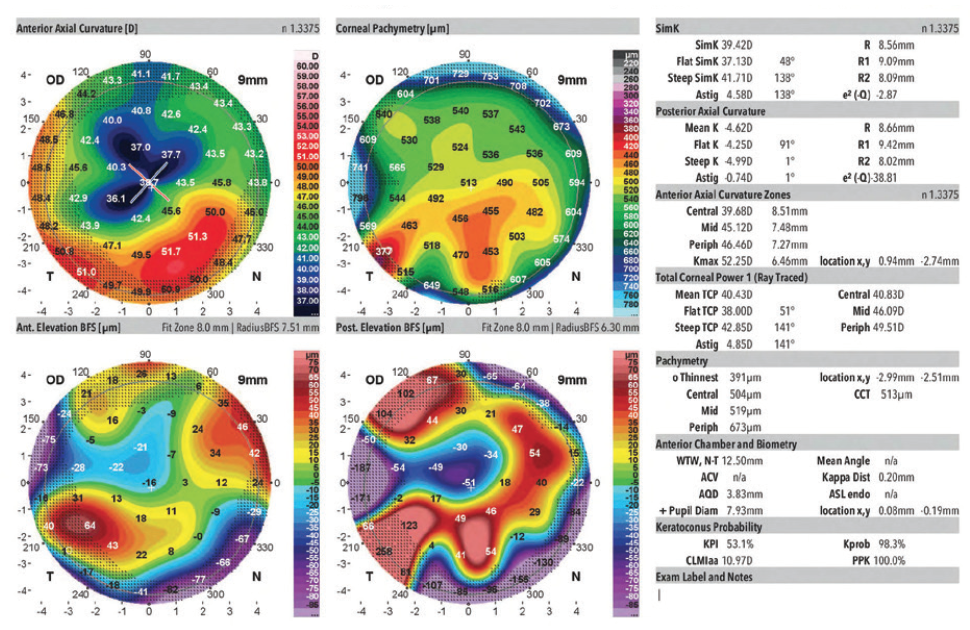
Figure 3. Measurements of the right eye obtained 3.5 years before cataract surgery on the left eye.
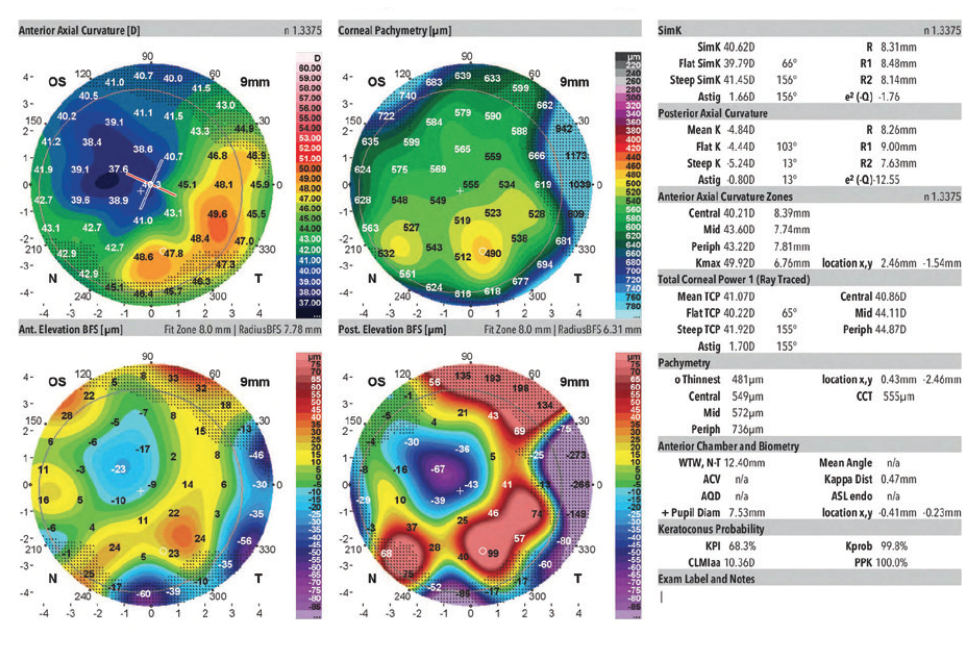
Figure 4. Measurements of the left eye obtained 3.5 years before cataract surgery on that eye.
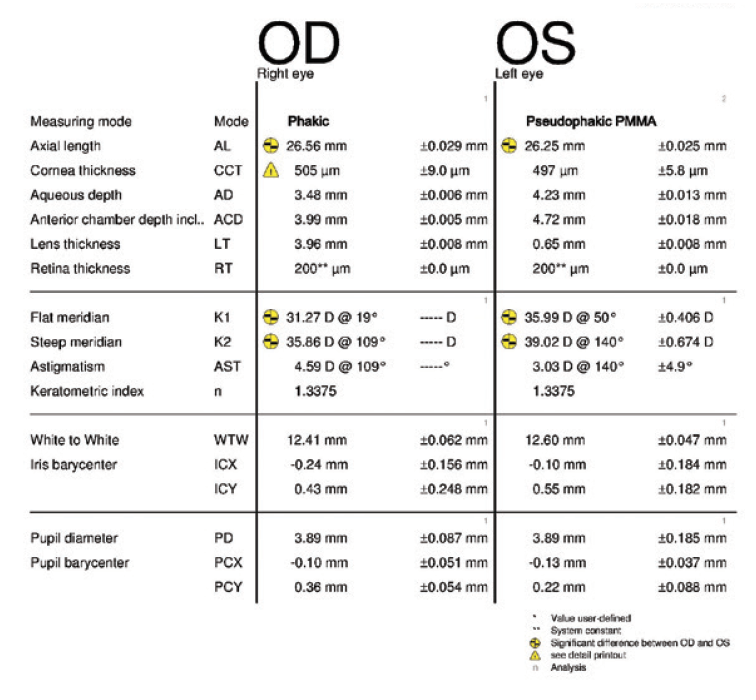
Figure 5. Measurements taken 3 months after cataract surgery on the left eye.
Two months after surgery, the left eye had a refraction of -3.75 -0.75 x 045º = 20/25-2. The cup-to-disc ratio was 0.9. Three months after surgery, the refraction was -2.50 -1.00 x 041º = 20/25 (Figures 5 and 6). Now, 2 weeks later, the refraction is -2.25 -1.00 x 055º = 20/25 (Figures 7–10). The right eye has a refraction of +4.25 -5.50 x 025º = 20/100.
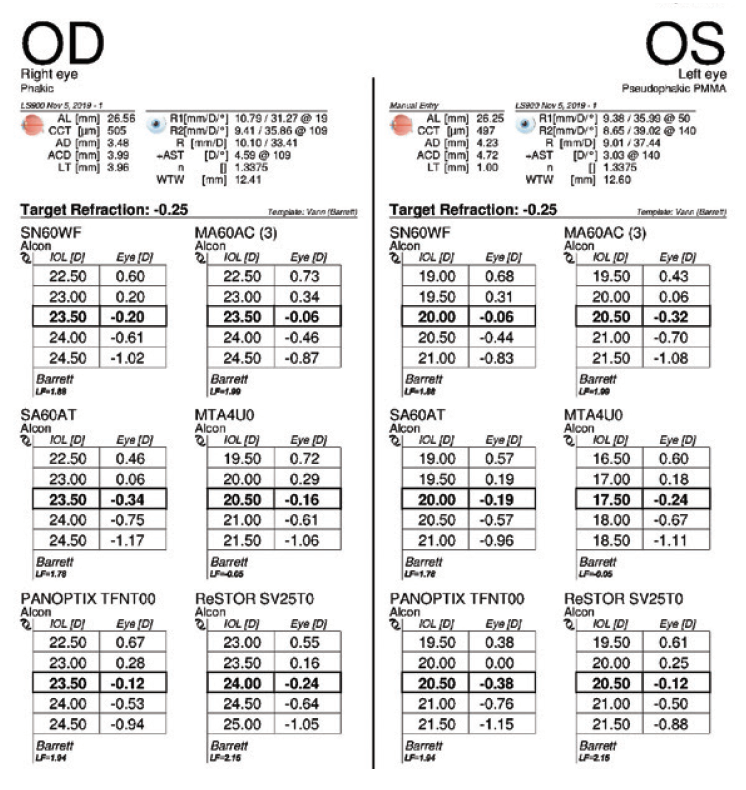
Figure 6. IOL power calculations performed 3 months after cataract surgery on the left eye.
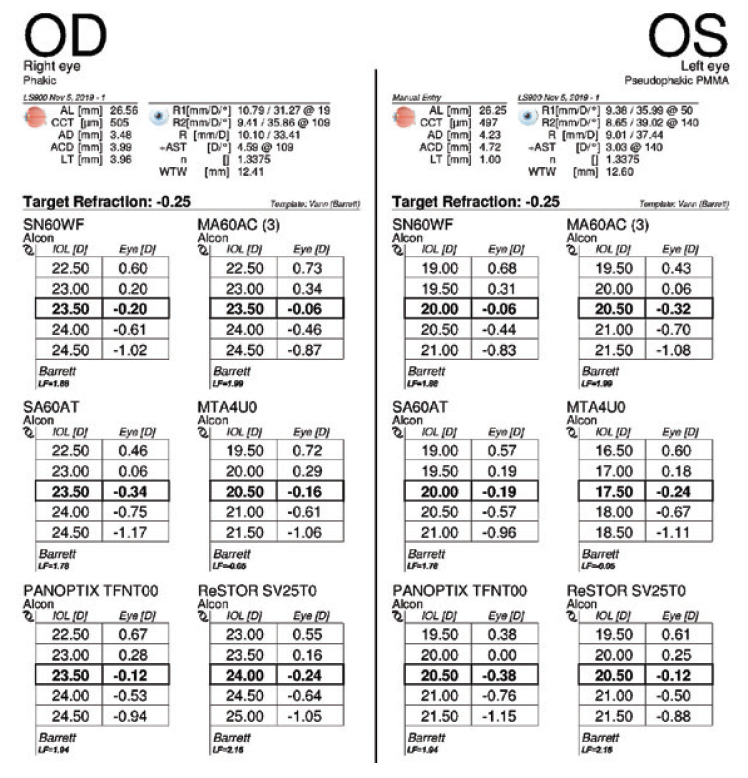
Figure 7. Measurements taken 3.5 months after cataract surgery on the left eye.
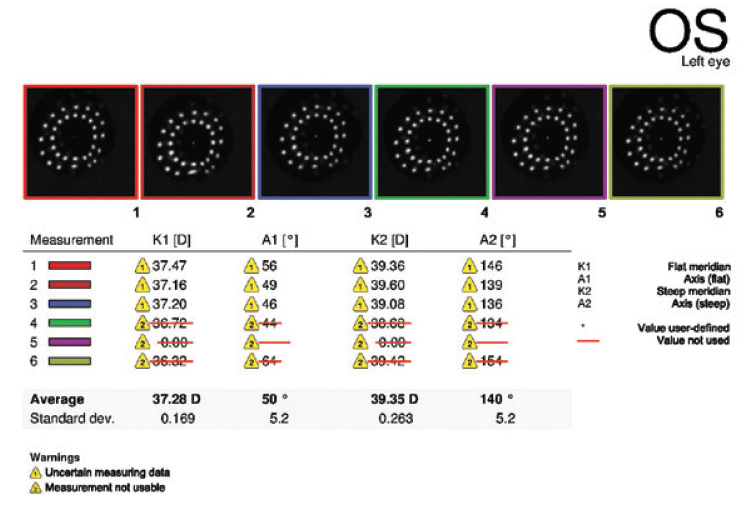
Figure 8. Keratometry analysis performed 3.5 months after cataract surgery on the left eye.
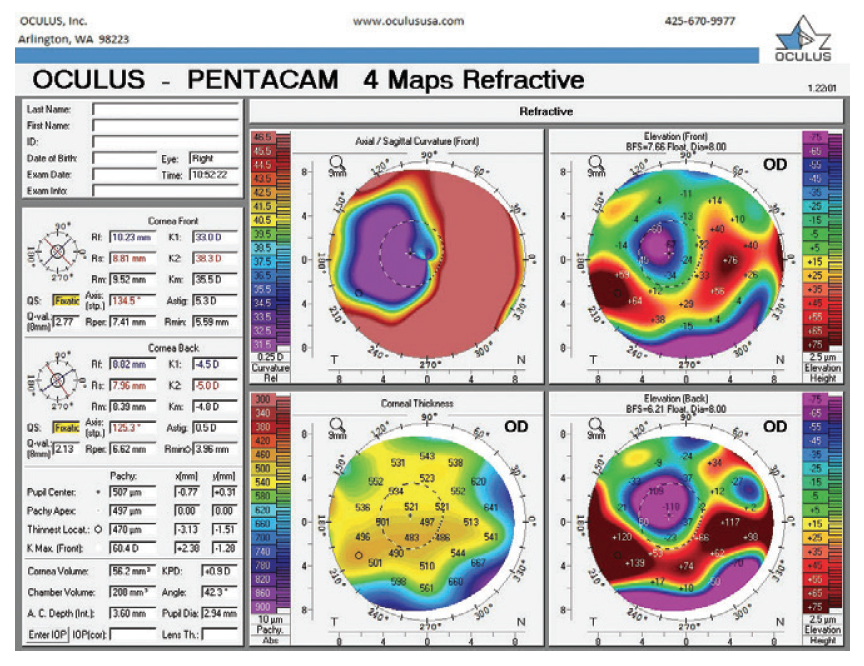
Figure 9. Refractive analysis of the right eye.
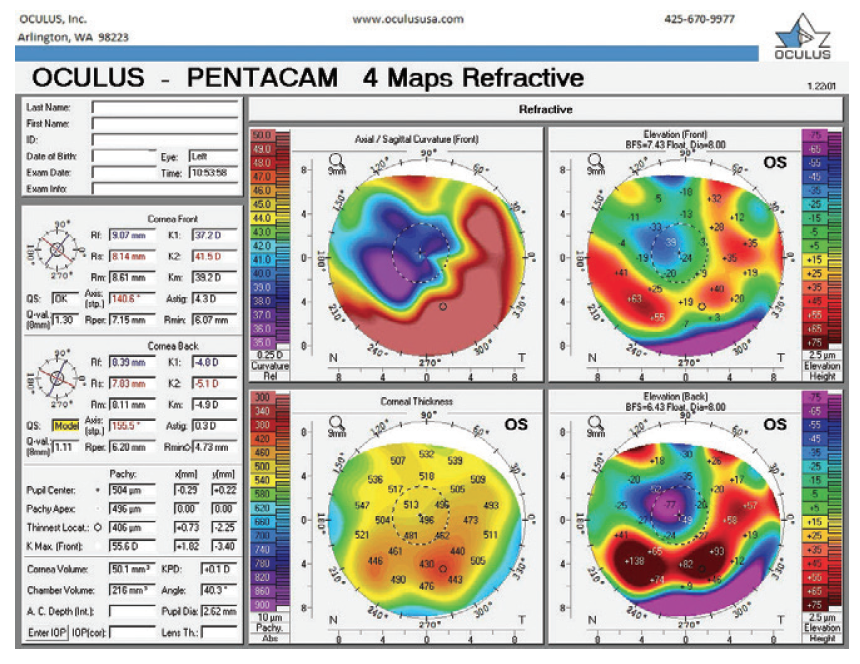
Figure 10. Refractive analysis of the left eye 3.5 months after cataract surgery.
The patient is currently wearing a hybrid contact lens (SynergEyes GP II, SynergEyes) in her right eye and no contact lens in her left eye. Her glaucoma surgeon wants her to discontinue contact lens wear to protect the filtration device that will be placed when she undergoes cataract surgery on the right eye, which has an irregular cornea and 9.00 D of astigmatism.
The cornea of the left eye has nearly returned to its preoperative state in terms of reliability for IOL power calculations, but the patient continues to experience refractive fluctuations and a myopic shift throughout the day. She is tolerating monovision but desires better distance vision in her left eye. Specifically, she has requested an IOL exchange for a toric lens with a target of distance vision in her left eye.
How would you address the corneal astigmatism in her right eye while respecting her inability to wear a contact lens after surgery? How would you address her desire to be dependent on reading glasses only?
—Case prepared by Alan N. Carlson, MD

DOUGLAS D. KOCH, MD
This is another in a long line of RK cases that are so discouraging for patients and doctors alike. There are two issues here.
No. 1: What can or should be done to improve UCVA in the left eye? Do nothing. We don’t know if the refraction is stable because the patient experienced a hyperopic shift in the past. Also, we don’t know how surgery on the right eye will turn out.
No. 2. What should be done with cataract surgery for the right eye? This eye could become myopic postoperatively, and then both eyes would work well together. There are two other reasons to avoid an IOL exchange in the left eye. First, another surgery could impair the glaucoma filter. Second, an IOL exchange will not blunt the diurnal fluctuations. One could consider performing epithelium-on CXL to blunt the refractive fluctuations.
Here is what we know about the right eye: BCVA is 20/100, and corneal measurements show more than 4.00 D of irregular astigmatism, with marked flattening temporally and steepening inferonasally. The most recent measurements with the Lenstar could not obtain a corneal reading because there is mire distortion and dropout in the raw mire images. It would be helpful to know the following two more things.
No. 1: What is the visual acuity with a contact lens in place? The answer will give some indication of how much of the vision loss is corneal versus lenticular in nature.
No. 2: What do the RK cuts look like? I suspect that the severe asymmetry in corneal power readings can be explained by gaping of some of the RK incisions.
The bottom line is probably that the cornea of the right eye will not provide visual acuity that is acceptable to the patient. The best optical solution for the right eye is almost certainly a scleral lens. One could determine whether the glaucoma surgeon would be comfortable with the option of cataract surgery combined with placement of a canal stent or some form of canaloplasty, which would avoid creation of a bleb. A nontoric IOL could then be implanted, and the patient could wear a scleral lens after surgery. It would be advisable to determine preoperatively if, in fact, a scleral lens will work for her.
If (as I suspect) the RK incisions are gaping, the cornea is a major cause of the current BCVA, and glaucoma filtration surgery is required, then there are three options.
No. 1: Topography-guided PRK. This may help if there is not a lot of incisional gaping, but I suspect that is not the case here. This procedure could be combined with CXL.
No. 2: Close the gaping incisions with 10-0 or 11-0 polyethylene terephthalate sutures (Mersilene, Ethicon). A ring or qualitative Placido device could be used to monitor suture tension, and the goal would be a slight overcorrection. If this strategy improves quality of vision, the sutures can be left in permanently, as they often do not biodegrade. One could then proceed with cataract and glaucoma filtration surgery. Sometimes this approach works, but, unfortunately, more often it does not.
No. 3: Penetrating keratoplasty. This is the option that I usually recommend as the initial step in this setting if contact lens wear is not feasible. If possible, I wait a year or more postoperatively to remove the graft sutures before proceeding with cataract surgery. Glaucoma surgery could be performed at any point.
The physician must help set realistic expectations with the patient about her vision and the complicated path ahead to treat the right eye.

ANDREW M.J. TURNBULL, BM, PGDIP CRS(DIST), FRCOPHTH
At the outset, this patient should be informed that refractive predictability is lower than usual, she may need spectacles postoperatively, and the clinical priority is preventing further glaucomatous loss of vision. Before considering any intervention, it is important to remember that the glaucoma in the left eye is severe with a cup-to-disc ratio of 0.9 and presumably advanced visual field loss. Appropriate expectations and informed consent are essential.
As an aside, it is curious that old biometry was used for the left eye. The postoperative Lenstar measurements show significantly steeper keratometry, which may reflect a change that occurred prior to surgery. To further elucidate the origin of the myopic refractive surprise, I would like to know what IOL calculation method was employed. I would not use intraoperative aberrometry because it is less reliable after RK owing to fluctuant biometry intraoperatively.1,2 Using the updated keratometry readings and Barrett True K formula, a lower-powered (19.50 D) SN60WF IOL would have been recommended.
To manage the refractive error in the left eye, implanting a piggyback lens based on her current refraction would be a less-invasive and more accurate option than an IOL exchange. The Barrett Rx formula would be useful in this regard, and a Sulcoflex toric IOL (Rayner, not available in the United States) would be my preferred option. Laser vision correction is possible after RK, but I would not consider it here because of the already flat cornea, advanced glaucoma, and bleb. I would remind the patient that any intervention could worsen her glaucoma and even cause optic nerve wipeout. Spectacles would be the safest option.
The glaucoma in the right eye is moderate and, one hopes, manageable with treatment or a device that does not require a bleb. This approach might permit ongoing use of a contact lens to control the irregular astigmatism, in which case I would implant a nontoric IOL. If the patient cannot wear a contact lens postoperatively, I would implant a toric IOL to reduce the regular astigmatic component as much as possible. Either way, I would base IOL power calculations on updated biometry and the Barrett True-K Toric Calculator (RK algorithm), ideally with refractive history. This has been shown to provide the greatest accuracy in this context.3
This toric calculator is beneficial even when implanting nontoric IOLs because it enables:
- Awareness of anticipated postoperative residual astigmatism rather than just spherical equivalent;
- Use of the K Calculator, which can improve outcomes by combining keratometry from multiple devices; and
- Consideration of surgically induced astigmatism.

ROBIN VANN, MD
The extreme corneal irregularity in both eyes is further complicated by the patient’s inability to wear a contact lens postoperatively because of the filtration device implanted by the glaucoma surgeon. Corneal power varies widely across the measurement modalities, with the average reading for the right eye ranging from 33.00 to 38.00 D. The left eye seems much more stable with less astigmatism.
Addressing the astigmatism in the right eye with a lens implant would leave the patient with extreme higher-order aberrations because of the 10.00 D difference in each meridian. Instead, I would focus on addressing the patient’s spherical goals by using the ASCRS IOL Calculator for Eyes With Prior RK and the Pentacam PWR_SF_Pupil_4.0 mm Zone method for guidance (Pentacam, Oculus Optikgeräte). I would prepare the patient for the likelihood of staged repeat surgeries to achieve her ultimate refractive goal.
Given the variability of the cornea in the unoperated right eye and the potential for further flattening after glaucoma filtration surgery, I would focus on performing an IOL exchange in the left eye, where a refraction vergence formula such as the Holladay R is more likely to achieve her goal of uncorrected distance visual acuity. I would use her glasses refraction to guide astigmatism management in that eye rather than biometric measurements that have difficulty estimating the astigmatism in such an irregular cornea. Once the left eye stabilized, I would turn my attention to the right eye.

DISCUSSION: ALAN N. CARLSON, MD
An increasing number of patients who previously underwent vision-correcting refractive surgery require cataract surgery. Of this population, post-RK patients pose unique challenges. To start with, records from the time of their RK procedures are rarely available. Moreover, these patients often have unstable corneas and irregular astigmatism, and intraoperative aberrometry is less reliable for IOL power selection and astigmatism management in these eyes. Furthermore, not only do these patients have high expectations, but I find that they tend to be more accepting of risk than post-LASIK patients. If I were to tell a post-LASIK patient that my success rate is 95%, I would be unsurprised to be asked about the other 5%. In contrast, I remember telling a post-RK patient that there was about a 30% likelihood that I could surgically achieve the desired goal, and the patient immediately wanted to know how soon surgery could be scheduled.
Many patients view RK as a procedure that changed their lives for the better. They have heard that cataract surgery can provide a similar, life-enhancing experience, and they do not fully understand how challenging their history of RK makes cataract surgery. Setting realistic expectations and establishing thorough documentation in their medical records prior to surgery are essential to avoiding postoperative disappointment and a perception that a complication occurred. It is important to emphasize that the recovery period may be prolonged, particularly if the cornea is unstable.
1. Canto AP, Chhadva P, Cabot F, et al. Comparison of IOL power calculation methods and intraoperative wavefront aberrometer in eyes after refractive surgery. J Refract Surg. 2013;29(7):484-489.
2. Zhang F. Optiwave Refractive Analysis may not work well in patients with previous history of radial keratotomy. Am J Ophthalmol Case Rep. 2018;10:163-164.
3. Turnbull AMJ, Crawford GJ, Barrett GD. Methods for intraocular lens power calculation in cataract surgery after radial keratotomy. Ophthalmology. 2020;127(1):45-51.




