
The ocular surface is the first refracting surface of the eye, so an optimized ocular surface is an important element in determining accurate biometric measurements and performing precise IOL power calculations. In short, optimizing ocular surface integrity prior to cataract surgery leads to better outcomes.
Average corneal power and magnitude and meridian of astigmatism can all be affected by the state of the ocular surface. Ocular surface disease (OSD), in particular dry eye disease (DED), can distort the ocular surface, inducing corneal astigmatism and affecting preoperative keratometric and biometric measurements.
If we physicians want to optimize postoperative quality of vision, mitigate complaints of visual fluctuation or discomfort, and avoid or minimize refractive postoperative surprises, we must do everything we can to manage DED preoperatively. Doing so will help us to achieve better objective and subjective outcomes.
When I prepare a patient for cataract surgery and it is apparent that a corneal issue such as moderate or severe DED, anterior basement membrane dystrophy, or an epithelium-based irregularity exists, preoperative treatment becomes a priority. In my experience, at least 5% of patients who are scheduled for refractive cataract surgery present with epithelial basement membrane dystrophy or other nodular degenerative changes that first require superficial keratectomy.
Case Study No. 1: Herpetic Stromal Keratitis
by Paul M. Karpecki, OD, FAAO

A man in his mid-40s was referred to me by an optometric colleague. The patient is a police officer and was having night vision problems that interfered with his ability to carry out his duties. His visual acuity was 20/30 OD upon presentation. He was diagnosed with herpetic stromal keratitis (Figure 1).
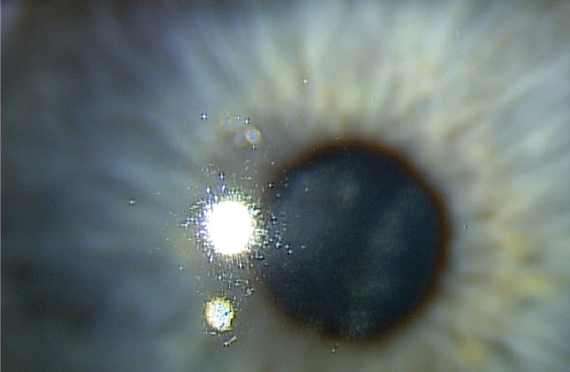
Figure 1. Patient presented with herpetic stromal keratitis.
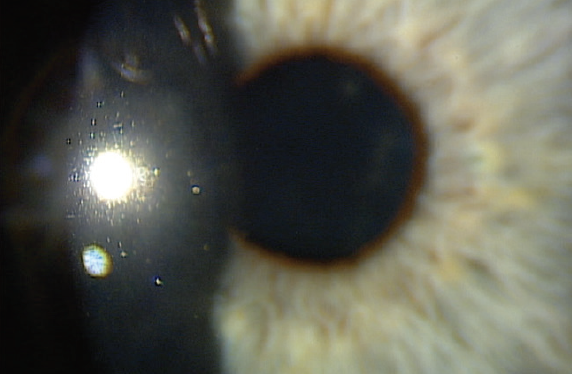
Figure 2. One day after AM application.
I placed a cryopreserved amniotic membrane (AM; Prokera, BioTissue) in his right eye in the office. The self-retaining cryopreserved tissue remained in place for 5 days. At 1 month, the cornea was clear, and visual acuity was 20/20 OD (Figures 2 and 3).
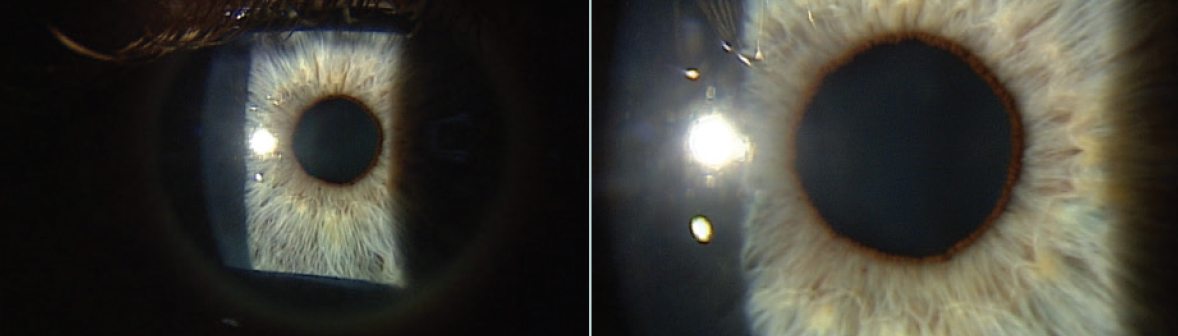
Figure 3. At 1 month after AM application, visual acuity was 20/20-2 OD and 20/15 OS.
TREATMENT TRIAGE
For patients with OSD, my first-line treatment is a steroid taper using either compounded preservative-free dexamethasone 0.025% for 3 to 4 weeks or loteprednol etabonate gel (Lotemax, Bausch + Lomb). The exception to this approach is for patients with glaucoma or any other instance in which elevated IOP is a concern. The regimen also includes frequent ocular lubrication and an oral omega-3 fatty acid supplement. When the patient returns for follow-up, if central corneal staining has not improved, I know that I am dealing with more severe, recalcitrant OSD.
In patients with recalcitrant DED that does not respond to these first-line treatments, or in those who require superficial keratectomy, I consider using amniotic membrane (AM) to expedite healing and reepithelialize the cornea so that optimal corneal curvature measurements can be obtained.
TWO TYPES OF TISSUE
Two types of AM are used for ophthalmic purposes: cryopreserved and dehydrated. Cryopreserved AM (Prokera, BioTissue) is kept frozen until shortly before use. Dehydrated AM (AmbioDisk, Katena Products; and BioDOptix, Derma Sciences) is stored at room temperature and rehydrated just before use.
The cryopreservation process allows cryopreserved tissue to retain heavy chain hyaluronic acid and PTX3, a biologic matrix that is responsible for the tissue’s antiinflammatory and regenerative healing properties.1 AM tissue that is dehydrated does not retain this crucial compound.2
Prokera has been cleared by the FDA and is indicated for protection, wound healing, and antiinflammatory applications. Dehydrated AM products have no formal medical designation from the FDA, and their only indication is for wound coverage.
Case Study No. 2: Severe Epithelial Basement Membrane Dystrophy
by Elizabeth Yeu, MD
A 67-year-old white woman presented for cataract evaluation in the right eye. The patient reported no pain, irritation, redness, or foreign body sensation in the eye. She had fluctuating vision that improved with a lot of blinking. Slit-lamp examination revealed that the patient had severe epithelial basement membrane dystrophy in both corneas (Figure 1).
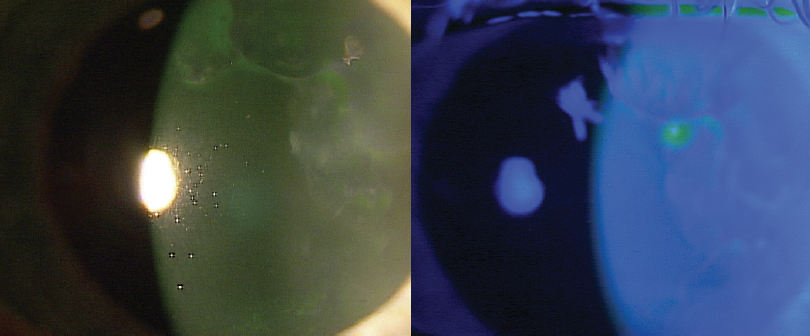
Figure 1. On presentation, the patient had severe epithelial basement membrane dystrophy in both eyes.
I performed a superficial keratectomy and placed self-retaining cryopreserved amniotic membrane (AM; Prokera, BioTissue) in the right eye (Figure 2). I prescribed a drop regimen of moxifloxacin and prednisolone acetate 1% three times daily for the duration of the AM, then prednisolone acetate 1% twice daily for 3 weeks.
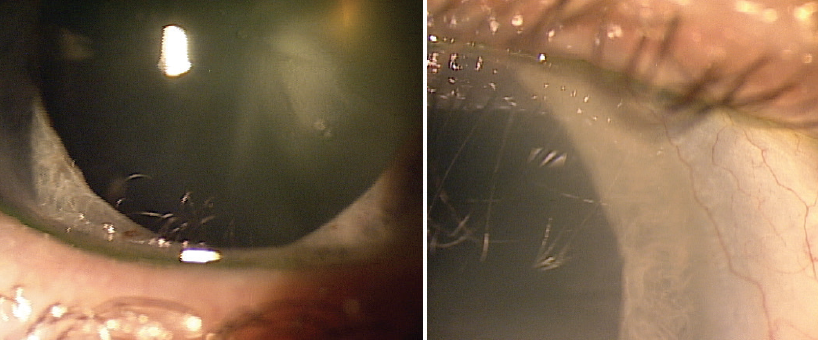
Figure 2. A superficial keratectomy was performed, and cryopreserved AM was applied to the right eye.
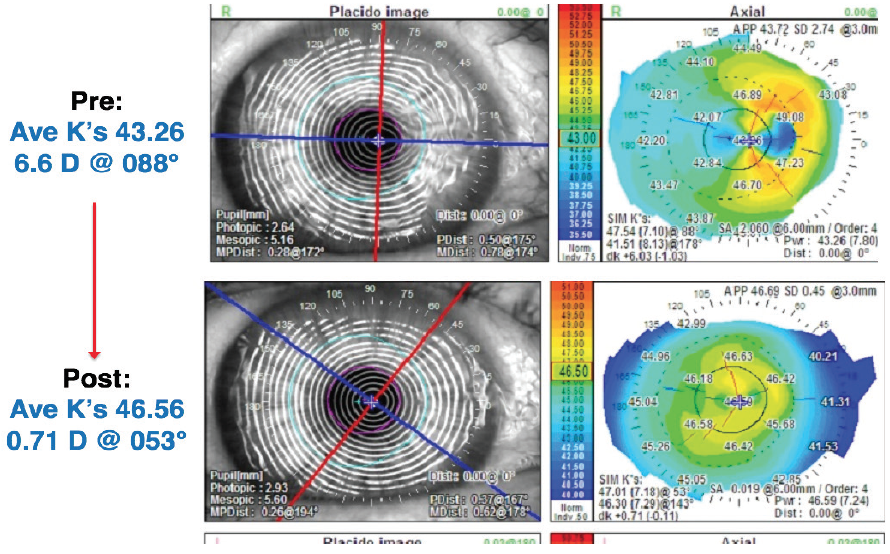
Figure 3. Corneal topography before (top) and 6 weeks after AM placement.
The cornea had fully reepithelialized in 5 days. Topography was performed 4 and 6 weeks after the AM placement to ensure stability of the corneal topography (Figure 3). These images revealed improvement in the corneal surface. Topographic astigmatism decreased from 6.60 D at 88° to 0.71 D at 53°. Average corneal power had also changed dramatically, from 43.30 to 46.58 D. The magnitude and meridian of astigmatism also changed. Central corneal power changed by 3.00 D.
Had I performed surgery based on initial measurements, the patient would have experienced a refractive surprise of greater than 3.00 D based on central corneal power.
Two months after the superficial keratectomy and Prokera placement, cataract surgery was performed uneventfully. One month after surgery, the patient’s UCVA was 20/25, and the manifest refraction was -0.50 +0.50 x 045º = 20/20.
WHY CRYOPRESERVED?
A growing body of literature supports the therapeutic capabilities of cryopreserved AM. In a retrospective study of approximately 100 patients with moderate to severe DED who were not responding to maximum conventional therapy, 88% of patients demonstrated improved corneal staining scores and notable reduction in the severity of symptoms after treatment with cryopreserved AM.3 Emerging research also suggests that, in addition to encouraging active healing, cryopreserved AM influences corneal nerve regeneration.4
In my experience, using self-retained cryopreserved AM to optimize the ocular surface prior to cataract surgery is preferable to using dehydrated AM. There are a few reasons for this observation. Cryopreserved AM is tissue-like, and the Prokera product is supplied on a self-retaining PMMA ring that lies outside the cornea and rests in the fornices. Upon the device’s removal, the ocular surface is extremely clean. Cryopreserved AM facilitates expedited healing compared with dehydrated AM, and this helps me get proper measurements sooner and speeds the process of getting patients to surgery.
Also, for patients with a corneal defect, cryopreserved AM is the only AM product with FDA clearance for therapeutic indications to address the inflammation and potential corneal hazing risk associated with the defect.
Dehydrated AM products, by contrast, are not self-retaining. They must be used with an accompanying bandage contact lens to keep them in place. As we know, with any kind of contact lens therapy, because of oxygen permeability issues and changes in corneal curvature, patients must be out of the contact lens for a few days to a week before cataract surgery. If the aim of AM therapy is to treat and clean the ocular surface in order to get the best measurements possible, treatment should occur close to the date of surgery; therefore, using dehydrated AM would not be ideal.
CRYOPRESERVED AM IN PRACTICE
If I am using cryopreserved tissue without an associated superficial keratectomy, I perform the procedure in the lane. To prepare the eye, I cleanse the lids with povidone-iodine and numb the eye with topical proparacaine. While the patient is looking down, I insert the tissue into the superior fornix. Next, I have the patient look up, and I place the bottom of the PMMA ring in the inferior fornix. The procedure takes less than 2 minutes.
When cryopreserved tissue is used for a patient with DED before cataract surgery, I do not use adjunctive antibiotic because the cryopreserved AM has its own antibacterial properties. If I am using cryopreserved tissue after a superficial keratectomy, patients are generally using a combination antibiotic-steroid, which they continue using 2 to 4 times a day.
With cryopreserved AM, patients typically epithelialize after one treatment, in 5 to 7 days. With dehydrated AM, it is not uncommon for the tissue under the bandage contact lens to go astray or even to go missing after 1 or 2 days because fixation can easily be compromised. When this happens, the patient does not achieve full epithelialization, and another membrane must be placed.
DEHYDRATED AM IN THE LITERATURE
While my experience with amniotic membrane favors the use of cryopreserved tissue, there are some noteworthy examples in the literature that point to the benefits of dehydrated AM.5 For instance, a study by Allen et al looked at augmented dried AM versus cryopreserved AM and found that dried AM that is pretreated with trehalose or raffinose and the antioxidant epigallocatechin is superior compared to cryopreserved AM for primary corneal epithelial cell expansion.6 The authors stated that this optimized dried AM has enhanced structural properties and biochemical stability and that these traits make it stable as well as easily transportable.
NO REGRETS
I have never regretted using cryopreserved AM. There have been times, however, when I regretted not using it sooner. For instance, I have had patients who did not initially exhibit obvious risk factors for slow healing, but their eyes did not epithelialize to my liking with first-line therapy. In those cases, I immediately proceeded with placement of cryopreserved AM. Once the cornea cleared, I was able to achieve the necessary measurements, choose an ideal IOL power, and proceed with cataract surgery.
The accompanying case reports by Paul Karpecki, OD, FAAO, and me illustrate some of the ways cryopreserved AM can be used in the clinic.
1. Cheng AM, Zhao D, Chen R, et al. Accelerated restoration of ocular surface health in dry eye disease by self-retained cryopreserved amniotic membrane. Ocul Surf. 2016;14:56-63.
2. Cooke M, Tan EK, Mandrycky C, et al. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J Wound Care. 2014;23(10):465-476.
3. McDonald MB, Sheha H, Tighe S, et al. Treatment outcomes in the Dry Eye Amniotic Membrane (DREAM) study. Clin Ophthalmol. 2018;12:677-681.
4. John T, Tighe S, Sheha H, et al. Corneal nerve regeneration after self-retained amniotic membrane in dry eye disease. J Ophthalmol. 2017;2017:6404918.
5. Noureddin GS, Yeung SN. The use of dry amniotic membrane in pterygium surgery. Clin Ophthalmol. 2016;10:705-712.
6. Allen CL, Clare G, Stewart EA, et al. Augmented dried versus cryopreserved amniotic membrane as an ocular surface dressing. PLoS One. 2013;8(10):e78441.




