CASE PRESENTATION
The most common question I have heard from other doctors lately runs along these lines: “I have a postrefractive surgery patient on whom I performed cataract surgery, and now I have a refractive surprise. What can I do to fix it?” For my next few turns as section editor of this series in CRST, I have recruited experts to provide some answers.
Although the panel will be discussing fixes for refractive surprises, the goal is prophylaxis for future cases. How can ophthalmologists fine-tune cataract surgery planning in patients with a history of refractive surgery, and how can surgeons prevent postoperative problems with quality of vision? In each case, the presumption will be that no refractive information is available prior to the intervention. Following is the first case.
A 70-year-old man underwent eight-incision radial keratotomy (RK) without astigmatic incisions in 1992. The patient presented in 2017 for treatment of a posterior subcapsular cataract in his right eye. His medical history was significant for long-term use of fluticasone propionate nasal spray (Flonase, GlaxoSmithKline). The patient had mainly been wearing glasses but intermittently changed to soft contact lenses when playing golf. The UCVA in his dominant right eye was 20/100, and the manifest refraction in that eye was +4.00 +0.50 x 040º = 20/30. Glare visual acuity measured 20/60.
In the workup for cataract surgery, biometry was performed, and the surgeon used the online ASCRS IOL power calculator for prior RK to select the IOL (Figures 1–4). During cataract surgery, the ophthalmologist was unable to obtain useful analysis with the »ORA System (Alcon). The surgeon implanted a 28.50 D »SN60WF IOL (Alcon), with a postoperative target refraction of -0.50 D.
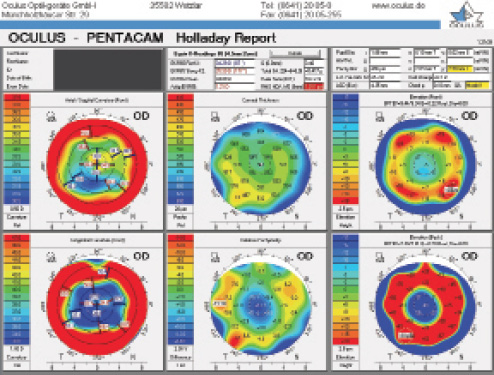
Figure 1. Holladay Report on the Pentacam (Oculus Optikgeräte) prior to cataract surgery.
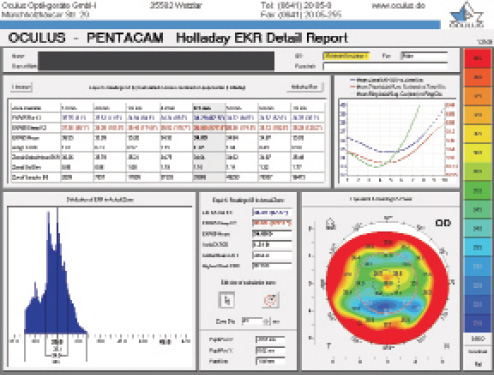
Figure 2. Holladay EKR Report on the Pentacam prior to cataract surgery.
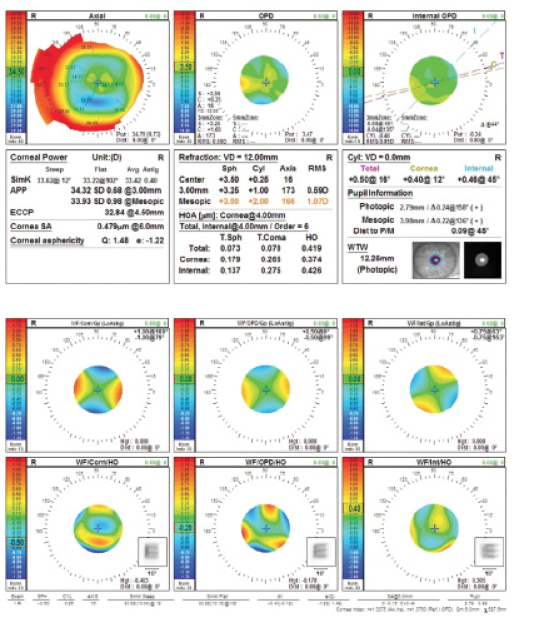
Figure 3. Preoperative measurements (top and bottom) with the OPD-Scan III (Nidek).
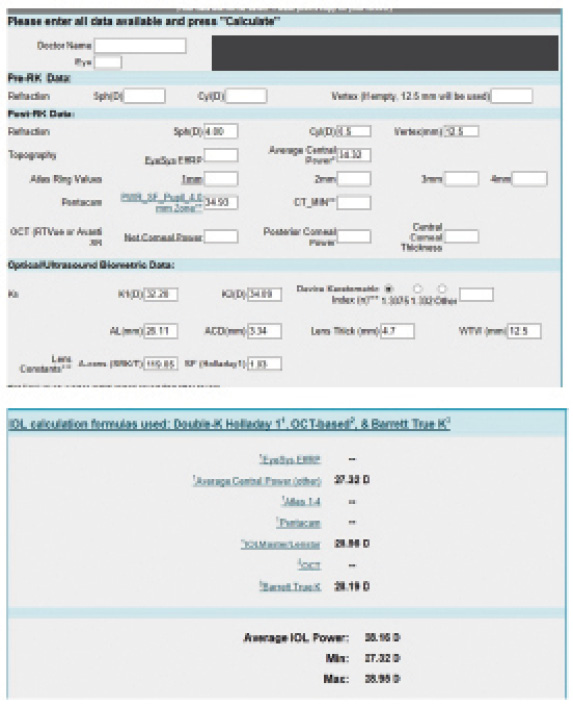
Figure 4. Preoperative calculations (top and bottom) using the ASCRS IOL power calculator for prior RK.
(Figures 1–4 courtesy of Karl G. Stonecipher, MD)
One year after cataract surgery, the patient’s UCVA was 20/150 OD. He wore a spectacle correction of -3.50 D. His manifest refraction was -3.75 +0.50 x 175º, and his cycloplegic refraction was -3.25 +0.50 x 175º. His cycloplegic refraction and BCVA were both 20/20.
How would you have calculated the IOL power for this patient, and what are his IOL options?
—Case prepared by Karl G. Stonecipher, MD

JACK T. HOLLADAY, MD, MSEE, FACS
This presentation is typical 26 years after eight- or 16-cut RK, with 4.25 D spherical equivalent hyperopia that will be greater in the morning than in the late afternoon. Keratometric measurements should therefore be obtained first thing in the morning to achieve an emmetropic result in the morning and a myopic shift throughout the day. Page 1 of the Pentacam Holladay Report for this patient, in the upper central panel, shows total spherical aberration of 0.417 µm (Figure 1), so an IOL with the highest negative asphericity, -0.27 µm, as available with the »Tecnis 1-Piece IOL (Johnson & Johnson Vision), would have been the best choice.
With 1.011 µm of root mean square higher-order aberration (HOA) wavefront error (a normal value is 0.37 µm), shown in the upper central panel of Figure 1, the quality of the patient’s cornea was poor, giving him a high likelihood of halos and glare with a monofocal IOL. A multifocal IOL was contraindicated. The broken spokes in the axial and tangential curvature maps confirmed the corneal irregularity, contraindicated a toric IOL because of the variability in the magnitude and axis of astigmatism, and suggested that intraoperative aberrometry would be almost impossible.
The thinnest part of the cornea is located 2.03 mm temporal and 2.90 mm inferior (8 clock hours) to vertex normal at 486 µm (upper right panel of Figure 1), which is 6.6% less than normal (relative pachymetry map at bottom center of Figure 1). Because this thinning is in the exposure zone of the cornea, it represents an incision that had thinned significantly and was likely to reopen at the time of cataract surgery. The front and back elevation maps are typical of post-RK eyes.
Page 2 of the Holladay Report shows the EKR65 (equivalent keratometry) in blue in the graph in the upper right-hand corner (Figure 2). It is worth noting that the blue curve (EKR65) begins centrally at 36.55 D, reaches a minimum of 35.84 D at 5 mm, and then gradually increases in power. The pupillary diameter of 1.88 mm is unusually small and suggests that using the 1- or 2-mm EKR65 values (36.55 and 35.99 D) would have been better than the standard 4-mm value (34.93 D) that is shown. The multiple power peaks in the graph in the lower left-hand corner and the EKR65 map in the lower right-hand corner of Figure 2 clearly show why a monofocal IOL was indicated with severe corneal irregularity.
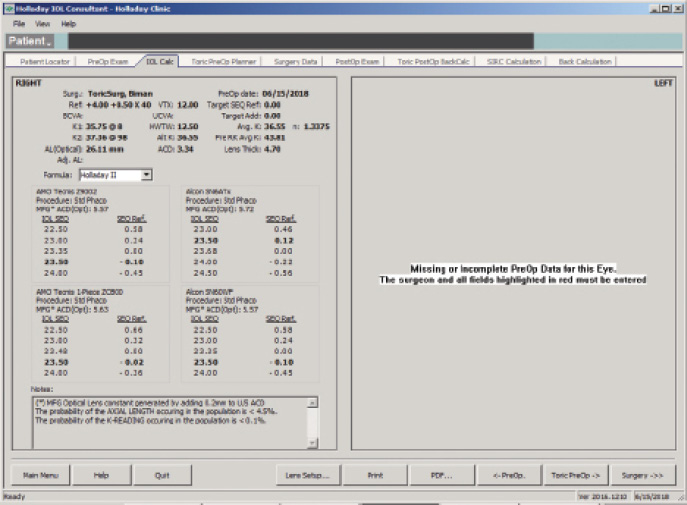
Figure 5. The Holladay IOL Consultant Software (Holladay Consulting) with the Holladay 2 formula using a mean EKR65 of 36.55 D.
(Courtesy of Jack T. Holladay, MD, MSEE, FACS)
The IOL calculation using the Holladay 2 formula for emmetropia with a mean EKR65 of 36.55 D (1-mm zone) yields a 23.50 D IOL (Figure 5). The predicted refraction using the Holladay 2 with the same EKR65 for a 28.50 D IOL (the one implanted) is -3.77 D (actual refraction was -3.75 +0.50 x 175º, Figure 6). The amount needed to reduce the current IOL power (28.50 D) may be found by changing the preoperative refraction to the current refractive surprise (-3.50 D), and the formula on the IOL calculation screen of the Holladay Refractive (R) shows that a -5.00 D change in power is needed to achieve emmetropia (Figure 7). This means that the current 28.50 D IOL should be reduced by 5.00 D, to 23.50 D, with an IOL exchange. After this correction, I would expect the patient’s BCVA with the aspheric monofocal IOL to be near 20/20 with 1.011 µm of corneal irregularity and mild to moderate reports of glare and halos.
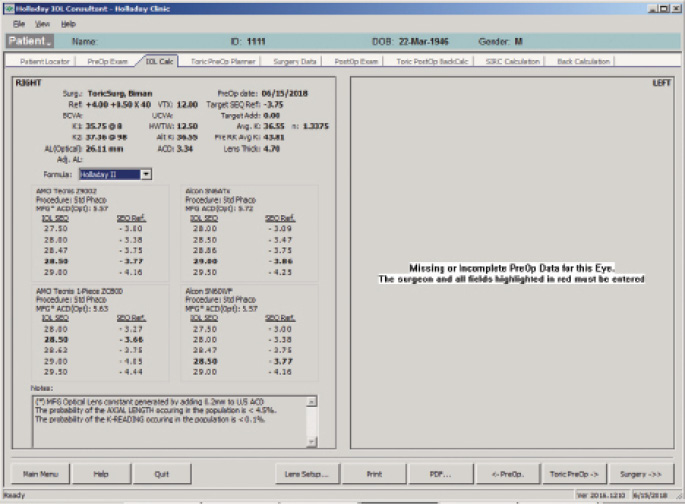
Figure 6. The Holladay IOL Consultant Software with the Holladay 2 formula using a mean EKR65 of 28.50 D.
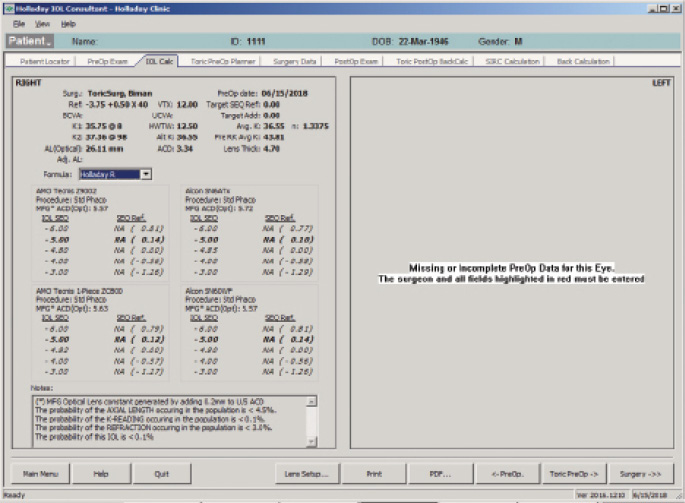
Figure 7. The Holladay IOL Consultant Software with the postoperative refractive surprise using the Holladay R formula.
(Courtesy of Jack T. Holladay, MD, MSEE, FACS)

DAVID COOKE, MD
I find cataract surgery outcomes to be most unpredictable in post-RK eyes. The most important thing I do preoperatively in these cases is to set reasonable expectations. I explain to patients that they will likely need a second operation. In cases like this one, RK is the gift that keeps on giving. Post-RK eyes of this sort generally continue to gain hyperopia, even after cataract surgery. I therefore intentionally aim for a -1.00 D result in hyperopic post-RK eyes like this one.
Not surprisingly, several of my post-RK patients have had unintended myopia after cataract surgery, similar to this case. I encourage these patients to endure their myopia because it will not last forever. They do not always agree, in which case I reoperate. My procedure of choice is an IOL exchange. I am concerned about the additional corneal flattening that would be produced by a PRK enhancement. I no longer recommend using piggyback IOLs because, in my experience, they cause too many problems with posterior iris chafing and there is a potential for pigmentary glaucoma in the future. I avoid toric IOLs in these cases because of the inability to define an axis, as indicated in the Holladay Report for this patient.

BRET L. FISHER, MD
Treating patients with a history of RK can be challenging; most will be highly motivated to become or continue to be spectacle independent, but, at the same time, they will likely be unaware of the special challenges they pose to the cataract surgeon. I discuss with these patients the difficulty of achieving refractive accuracy in eyes with a history of refractive surgery, and I have them confirm their understanding of the discussion by signing a special informed consent document.
I am comfortable implanting a standard monofocal, a toric, or an accommodating IOL in post-RK eyes, although in this case I would have used a monofocal or accommodating IOL because of the irregular astigmatism evident on preoperative corneal testing. I use the ASCRS calculator, as was done in this case, and I generally base my initial IOL power selection on the Barrett True K formula result. For this patient, my initial choice would have been a 28.00 D IOL. I perform intraoperative aberrometry during cataract surgery on all eyes that have undergone refractive surgery. In some post-RK eyes, especially those with more incisions or small optical zones, it is not possible to get a final reading on capture, but by viewing the live streaming data, it is usually possible to estimate the IOL power and compare it to the preoperative estimate. Using this method, I have had few significant refractive surprises in post-RK patients.
To manage this patient’s refractive surprise, PRK would be a reasonable approach, but an IOL exchange could also be considered because of the low amount of residual refractive astigmatism. To plan for an IOL exchange, I would use the known refractive result and then back-calculate using the Holladay R formula to arrive at the new IOL power. I would exercise caution during the exchange procedure, however, because I have found a continuing and increased tendency for previous RK incisions to open during continued manipulation of the eye. The need to suture one of these incisions can spoil the refractive result and add unnecessary complexity to some cases.

UDAY DEVGAN, MD
This patient received a 28.50 D IOL and ended up with a postoperative refractive error of approximately -3.00 D spherical equivalent, suggesting that a 24.00 D IOL would likely have produced a plano result. Each diopter at the spectacle plane is about 1.50 D at the IOL plane, so the -3.00 D result suggests that the IOL power is 4.50 D too high. In retrospect, the best preoperative information would have been obtained by using the Holladay EKR value of 35.00 D for the cornea. That approach might not work well in all post-RK eyes, however, and therein lies the dilemma.
I would only use a monofocal IOL for this patient because of his corneal irregularities and HOAs. The WF/Corn/HO printout from the Nidek OPD scan tells the story: The convolved E is highly distorted by the corneal HOAs (Figure 3). This cornea is not a great candidate for any IOL with diffractive rings, whether multifocal or extended depth of focus. A PRK to address the -3.00 D refractive surprise would help achieve a plano result in this case, but it would also further flatten an eye that is already very flat.

WHAT I DID: KARL G. STONECIPHER, MD
Once the patient’s refraction stabilized after cataract surgery, I performed transepithelial PRK based on a contact lens trial (a video of the procedure can be viewed below). The patient desired monovision correction, and he has done well postoperatively.




