
Noel Alpins, AM, FRANZCO, FRCophth, FACS
There are several ways to correct astigmatism surgically, including the use of incisions, ablations, and toric implants; but there is only one way to analyze changes in astigmatism in an intuitive way with a common standard, regardless of the mode of correction. That way is known as vector analysis, and I have been a champion of its use in postoperative analysis of surgical astigmatic results for several decades.
At the simplest and most practical level, determining whether an astigmatic treatment over- or undercorrected, or whether it was on- or off-axis in a clockwise or counterclockwise direction, allows one to systematically analyze errors in a group of patients in order to make future adjustments for better results.
People often confuse astigmatism and its measurement by a clinical device with vectors and their calculation because they share the same units of measurement: diopters and degrees. Examining each of these alone can be useful in some respects, such as to determine how much any preoperative astigmatism may have decreased or increased; yet comparing postoperative to preoperative astigmatism provides no insights as to how the process misperformed. However, when we consider both magnitude and orientation of astigmatism when we analyze change from pre- to postoperative, a vectorial analysis can be provided, rather than just a scalar magnitude analysis.
Vector analysis is a perfection of mathematics. This type of analysis can be used as a benchmark for assessing the effect of surgery intended to reduce astigmatism. But it is more than this: Its parameters are intuitive for the surgeon, and it provides a rational basis for understanding the changes made and why any treatment may have failed to achieve its desired result.
Questions for the panel:
No. 1: How do you analyze your astigmatic outcomes postoperatively?
No. 2: Do you analyze astigmatism using both refractive and corneal parameters?

Jorge L. Alió, MD, PhD, FEBOphth
Method for analyzing astigmatic outcomes. I analyze my outcomes postoperatively in every case, no matter what method I am using to correct astigmatism: incisions, excimer laser, or toric IOLs (both phakic and pseudophakic). Preoperatively, I determine the astigmatic target based on the patient’s clinical refraction and anterior corneal topography with the Sirius topographer (CSO).
If the clinical outcome is good, my analysis ends at this point. If I have a mismatch of more than 0.50 to 0.75 D, and if the patient is clinically affected, then I analyze my outcome using pyramidal aberrometry on the Osiris (CSO) and the »KR-1W (Topcon), which combines both wavefront and topographic technologies. (The clinical effect on the patient varies. It is not the same to have a with-the-rule and an against-the-rule astigmatic error. In some presbyopes, there is some advantage in having a with-the-rule astigmatic outcome, as long as it is not more than 0.75 D.)
With the patient’s first eye, I try to understand the total behavior of the eye in terms of variables other than refraction—especially coma aberration. With the second eye, then, I can try to estimate how much of the eye’s total clinical astigmatism comes from the intraocular structures and how much comes from the anterior corneal surface. These devices allow me to separate internal from anterior corneal and total astigmatism.
Then, if the mismatching calls for an intervention, I apply the Alpins Statistical System for Ophthalmic Refractive Surgery Techniques (ASSORT) program. This is the most comprehensive method of all, as it provides an understanding of how much error was due to a mistake in incision creation (surgically induced astigmatism) or in toric lens alignment, and it indicates the correction that is needed.
If I am analyzing results with pseudophakic or phakic toric IOLs, then I like to use www.astigmatismfix.com, a program designed by David R. Hardten, MD, FACS. Based on cosine analysis of the astigmatism, it calculates the amount of IOL rotation needed and the residual refraction that can be expected. We have to consider the ocular residual astigmatism—a concept coined by Dr. Alpins and me—because in most cases we cannot correct astigmatism to a mathematical zero.1
After all this analysis, I understand what I have done and what has happened to my patient, and I can do my best to lead the patient to an adequate outcome as I plan further correction.
Use of refractive and corneal parameters. The clinical refraction is the most important measurement of refractive error because it is the outcome that is experienced by the patient. Having said that, to understand the background of the clinical refraction, especially in cases in which there was an error in correction, we need to determine the parameters of the anterior corneal surface, the posterior corneal surface (using the Sirius), and the lenticular astigmatism.
The concept of internal astigmatism, as analyzed by the KR-1W, includes both the lenticular and posterior corneal astigmatism. If a toric IOL (either phakic or pseudophakic) was implanted, then the toricity of the lens is known, and the rest belongs to the posterior corneal surface. I try to plan correction either by corneal or intraocular surgery (rotating the lens), as necessary. I always prefer in the case of toric IOLs to rotate the lens to the best axis, as it makes the most sense, is easy, and is clinically feasible if performed before the end of the second postoperative week.
For monofocal IOLs, I deal with residual corneal astigmatism with excimer laser surgery. My preference after cataract surgery is to use PRK, which is effective and minimally traumatic in the adult or elderly patient. I do not use incisional surgery in postoperative correction. My actions are guided by the clinical refraction but with insight based on the patient’s anterior corneal topography, which is used to modify the excimer laser ablation. I use the posterior corneal data only when calculating pseudophakic IOL power. I do not take posterior corneal astigmatism into consideration with phakic IOLs; I use only the clinical refraction and the anterior corneal topography data.
1. Alió JL, Alpins N. Excimer laser correction of astigmatism: consistent terminology for better outcomes. J Refract Surg. 2014;30(5):294-295.

George O. Waring IV, MD, FACS
Method for analyzing astigmatic outcomes. We analyze our postoperative astigmatic outcomes via serial manifest refractions after 1 month. When warranted, we utilize elevation-based tomography (»Pentacam, Oculus Optikgeräte), ray tracing (»iTrace, Tracey Technologies), and high-definition Shack-Hartmann wavefront technology (»iDesign, Johnson & Johnson Vision). We do this for both cornea- and lens-based astigmatism analysis.
Use of refractive and corneal parameters. For corneal vision correction, we primarily evaluate the corneal parameter as it was the only optical surface altered. For bioptics—cornea and lens simultaneously, inclusive of any limbal relaxing incisions and IOL—we primarily evaluate the cornea. For toric IOLs, we evaluate both corneal and IOL parameters. Additionally, for toric IOL analysis, we use www.astigmatismfix.com and the post-toric software on the iTrace.
We use these advanced diagnostics to aid in our decision-making algorithms, specifically with regard to how to approach residual astigmatism. This multifactorial process relies on successfully identifying the etiology, which then drives the most appropriate treatment. Residual astigmatism after a cornea-based procedure is typically caused by over- or under-treatment and is generally addressed at the corneal plane. The treatment is verified with corneal and wavefront parameters but is based on refractive (manifest) and wavefront guidance.
For residual astigmatism after toric IOL placement, the treatment is based on both refractive and corneal parameters and takes into account three things: (1) the correct target axis, (2) the correct target magnitude inclusive of posterior corneal astigmatism adjustment, and (3) the correct placement and effective lens position inclusive of intraoperative marking and postoperative effective lens position. The treatment plan is based on the etiology.
Professor AlPins Replies
The panelists have given detailed and comprehensive answers to the two questions, providing useful information for several clinical situations.
Professor Alió points out that with-the-rule astigmatism is a more favorable orientation for any unavoidable astigmatism remaining postoperatively, and this is a key element in surgical strategy. Treatment of the primary spherocylindrical ablation reduces any coexisting coma that may be secondary to irregularity. Aligning the cylindrical treatment close to the principal corneal meridian is likely to enhance the effect. Knowing the ocular residual astigmatism (ORA) prior to surgery to quantify internal (or nonanterior corneal) astigmatism is certainly a valuable exercise for any astigmatic treatment.
Recognizing and calculating the ORA preoperatively allows the surgeon to determine how much astigmatism can be corrected surgically and that eliminating all the astigmatism from the system in most cases is not possible. This is an essential element to address in patient counseling and surgical planning, as Professor Alió recommends, and can be effectively addressed using the method of vector planning. The total astigmatism of the eye as measured by manifest refraction has several components, including the anterior and posterior cornea, the crystalline lens, and the nonoptical component of perceptual astigmatism that makes up the ORA and is not measurable with an optical device. This subjective patient component can be responsible for refractive surprises both in excimer laser ablation surgery and with toric IOLs. The ASSORT software (www.assort.com) has a free refractive surprise calculator that includes the effect of the effective lens position (ELP) using the axial length and total corneal power to recommend toric IOL rotation (Figures 1 and 2). This can help to minimize postoperative refractive cylinder. It also includes the important vector analysis of the astigmatic correction by the toric implant to determine if the IOL’s toricity is the correct power. This is calculated by using the magnitude of error and correction index achieved and helps decide whether an IOL exchange would provide clinical benefit. This refractive surprise analysis is a hybrid vectorial analysis and uses refractive (postoperative) and corneal (preoperative) astigmatism values.
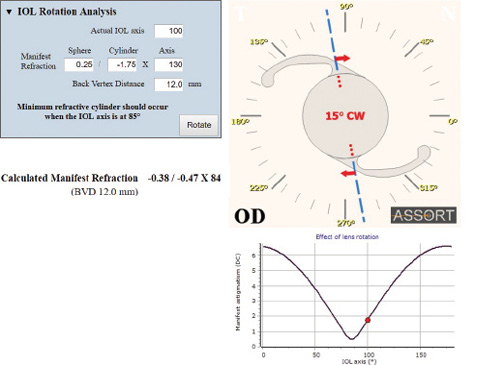
Figure 1. The display on the ASSORT toric IOL refractive surprise calculator showing the most effective rotation of the implanted toric IOL to minimize the refractive cylinder. In this example, a 15º clockwise rotation of the IOL would reduce the postoperative refractive cylinder from a magnitude of -1.75 to -0.47 D.
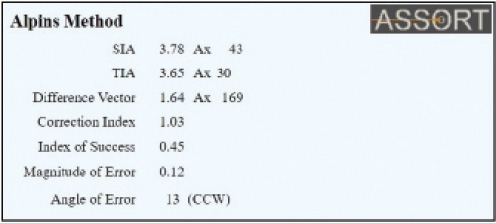
Figure 2. The vectorial analysis display on the ASSORT toric IOL calculator to determine whether the toric power of the implanted IOL is correct. This is determined by the Magnitude of Error (ME) and the Correction Index (CI). The ME displayed is negligible (0.12 D in this example). An ME of greater than 0.75 D may indicate that explantation of the current toric IOL is required to reduce the refractive cylinder, and that rotation would be ineffective. The CI here is 1.03, which indicates that the toricity of the implanted IOL is ideal.
As Dr. Waring says, there is useful information from either a separate corneal or refractive astigmatic analysis depending on the procedure that is performed. But any corneal or refractive procedure can benefit from both in parallel. For example, when an enhancement is required after spherocylindrical LASIK or PRK, any astigmatic over- or undercorrection should be determined separate from the spherical equivalent outcome. So calculating the correction index both with corneal and refractive parameters assists in any nomogram adjustment required for the retreatment of the remaining cylinder.




