Contemporary management of dry eye disease (DED) is predicated on three approaches:
1. Keep tears on the ocular surface longer.
2. Reduce local inflammatory response to downregulate inflammatory mediators.
3. Stimulate and remove mechanical blockade from the meibomian glands to clear the way for the production of meibum.
These steps, alone or in combination, help eye care providers to restore the production of physiologically normal tears—with their delicate balance of lipid, aqueous, and mucous components—in patients with DED. This is, of course, a simplification of what can be a multitiered, multipronged approach. Using punctal plugs and supplements to keep tears on the eye, prescribing pharmacologic agents to address inflammation, and getting the meibum flowing via a variety of mechanisms, however, are all fundamental approaches we physicians use regularly to treat DED at its source.
Recent clinical trials suggest that there may also be a role for mechanisms that increase tear production by the lacrimal glands. Stimulating them to increase aqueous production in eyes with aqueous-deficient DED might address the immediate problem and potentially effect long-term functional changes.
The use of neurostimulation to provide therapeutic benefit is well established in other areas of medicine. Many of us are familiar, for instance, with its use for the treatment of chronic pain and for rehabilitative purposes. There are also research applications in neurodegenerative disorders such as Alzheimer disease and epilepsy.
Now, researchers are exploring the use of neurostimulation for treating DED. Data from two clinical trials of the TrueTear device (Allergan), OCUN-009 and OCUN-010, were announced last year.1 These studies suggest that the device, which stimulates the lacrimal glands, effectively increases tear production in eyes with DED. Allergan acquired the device in its purchase of Oculeve in 2015.2 Allergan expects to submit premarket data to the FDA in the second half of this year.1
DATA AND PERSONAL EXPERIENCE
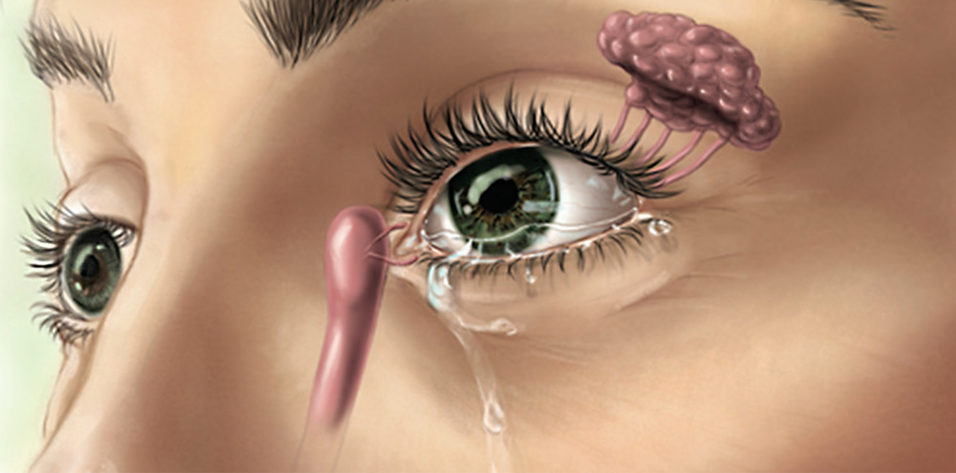
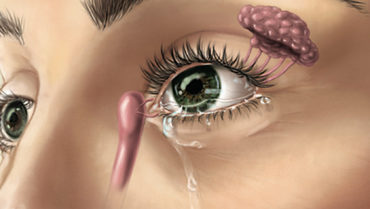
The TrueTear is a noninvasive, handheld, patient-administered device intended to increase tear production in patients with DED. The device has two prongs that, when inserted through the nose, contact the ophthalmic branch of the trigeminal nerve and stimulate cranial nerve VII, which innervates the lacrimal glands. Low-level electrical stimulation from the device stimulates the lacrimal gland, increasing tear production (Figure).
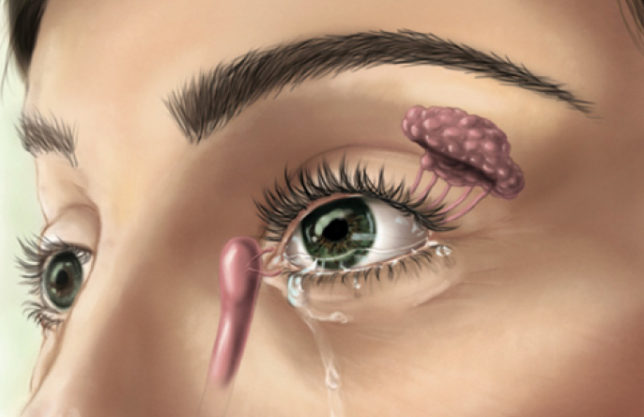
Figure. Diagram shows TruTear mechanism of action.
The immediate benefit associated with the TrueTear is easily understood: stimulation of the lacrimal gland addresses the acute problem in DED by providing the eye with a greater volume of tears. What is less well understood is whether use of the device may also effect long-term remodeling, in which case it may address the chronic nature of DED as well. Although the science has yet to be elucidated, it has been proposed that neurostimulation will aid in kick-starting the lacrimal system to perform in a more physiologic manner.
The clinical trial data made publicly available so far suggest that continued use of the TrueTear provides a durable benefit. In the prospective, single-arm, multicenter, open-label OCUN-010 study, patients were instructed to use the device for 180 days, and they were evaluated with Schirmer testing on days 0, 7, 30, 90, and 180. At all time points measured, patients demonstrated increased tear production over baseline.1
Because the device is easy to use, it is reasonable to assume that, once it becomes clinically available, the TrueTear will allow patients to achieve rapid and durable relief of their DED symptoms. I readily admit my skepticism when I first heard about the concept of neurostimulation for DED. Seeing a patient who told me she was able to read a book for the first time in 7 years after treatment with the device started to change my mind. When more patients reported similar outcomes to me, I became convinced that the TrueTear could be a powerful tool for patients experiencing all grades of DED.
CONCLUSION
DED is a multifactorial and complex disease. It is also a frustrating and complicated problem for patients. Too often, they have to take numerous steps to rehabilitate the tear film, including using warm compresses multiple times a day, taking omega-3 fatty acid dietary supplements, instilling eye drops, and making adjustments to their daily routines. In a sense, patients are replacing the burden of a disease that negatively affects their quality of life with multiple steps that negatively affect their quality of life. Over and above its effectiveness, the TrueTear offers the promise of simplifying treatment.
Questions about patient selection for the device remain to be answered. Because the TrueTear is still investigational, we cannot yet fully determine its clinical application. I suspect that the greatest potential application will be in individuals with aqueous-deficient DED, but I will not be surprised if the device helps patients with all forms of the disease. It also seems reasonable to think that restoring patients’ ability to produce their own healthy tears is the ideal strategy for restoring their ocular health.
1. Allergan announces positive pivotal trial results for Oculeve intranasal tear neurostimulator [press release]. Allergan. May 16, 2016. http://bit.ly/2nFJ6H8. Accessed March 15, 2017.
2. Allergan successfully completes Oculeve acquisition [press release]. Allergan. August 10, 2015. http://bit.ly/2noqCPm. Accessed March 15, 2017.