Until recently, the suprachoroidal space for glaucoma surgeons was a place for effusions to accumulate or, even more feared, a place for hemorrhage to occur in rare cases of hypotony. A look at the distant past, however, reveals that ophthalmic surgeons used to create cyclodialysis clefts into the space intentionally to lower IOP.1 The unpredictability of the unstented cleft approach eventually led to the development of alternative bypass procedures into the subconjunctival space.2-4 Technology evolves, and history informs modern innovations.
AT A GLANCE
• On July 29, 2016, the FDA approved the first stent that accesses the suprachoroidal space by insertion into the supraciliary space, the CyPass Micro-Stent.
• The suprachoroidal space avoids the more reactive fibroblastic healing response and complications associated with a subconjunctival bleb, yet it reserves conjunctiva for additional procedures in the future.
• The availability of suprachoroidal access devices will increase surgeons’ ability to tailor glaucoma therapy to each individual.
TRABECULAR OUTFLOW ACCESS DEVICES
The first ab interno trabecular microstent on the market (iStent; Glaukos) and an investigational Schlemm canal stenting device (Hydrus Microstent; Ivantis) both rely on an intact downstream outflow system to be effective. If episcleral venous pressure is high or if damage to the aqueous drainage system within the episclera is suspected, perhaps from long-term drug use or scarring, a trabecular meshwork/Schlemm canal stent presumably would not address the anatomical issue involved. Even with a few years of experience, it is still difficult for us to predict which patients will do well with the iStent, because our ability to know the outflow system’s status lags behind our ability to access it.
SUPRACHOROIDAL ACCESS DEVICES
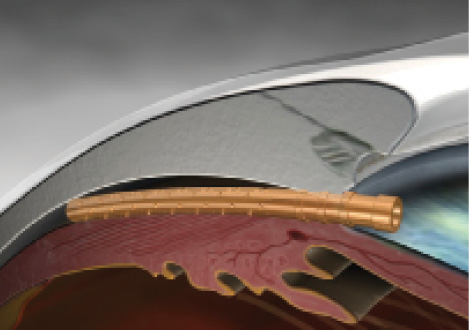
Figure 1. The CyPass Micro-Stent in place.
We will soon have new options that forgo the conventional outflow system and use the suprachoroidal space as a reservoir for aqueous flow to lower IOP. On July 29, 2016, the FDA approved the first stent that accesses the suprachoroidal space by insertion into the supraciliary space, the CyPass Micro-Stent (Alcon; Figure 1). The arduous 2-year pivotal study is available online and expected to be published in the October print issue of Ophthalmology.5 The CyPass is a 6.35-mm-long, polyimide, hollow, flexible, fenestrated tube with retention rings on the anterior chamber end. To conform to the curvature of the supraciliary space, the device is curved during ab interno insertion with a guidewire, after which the CyPass rebounds to a noncurved state in order to provide a stenting effect.
Glaukos is also developing a suprachoroidal bypass device, the iStent G3 Supra, and the company is currently recruiting patients in a multicenter US trial.
A TRANSFORMATIVE TECHNOLOGY?
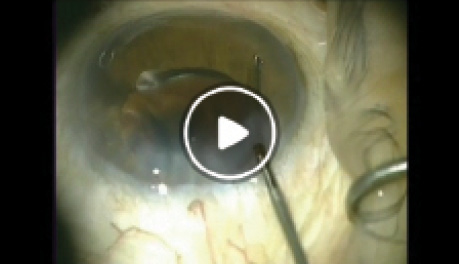
Watch it Now
E. Randy Craven, MD, demonstrates the implantation of the CyPass Micro-stent immediately after cataract surgery.
Will suprachoroidal access devices transform surgical care for US patients? Certainly, these devices will give us more choices and the ability to tailor our approach to each individual. Depending on study results, real-life experience, and ease of use, we surgeons are likely to gravitate initially toward what works best in our hands for our patients. As outflow imaging or functional testing becomes available, we will have more precision in our choice of the proper device for each patient.
WHY THE SUPRACHOROIDAL SPACE?
First, the suprachoroidal space avoids the more reactive fibroblastic healing response and complications associated with a subconjunctival bleb, yet it reserves conjunctiva for additional procedures in the future. Second, outflow through the suprachoroidal space is known to have a large gradient from the anterior chamber that drives aqueous there. Third, the space is accessible. Fourth, from our experience with drugs that pharmacologically enhance uveoscleral outflow via the increasing outflow through the suprachoroidal space, we already know that the effect is decreased IOP.
WHAT ARE THE RESULTS?
The research portfolio for the CyPass has involved numerous patients in Europe, with and without cataract surgery and with and without refractory glaucoma. In general, the results have shown a reduction in IOP of over 30% that was sustained for 2 years, with patients who have both refractory glaucoma and earlier-stage glaucoma experiencing approximately a 50% or greater reduction in the number of preoperative medications. Patients who had the CyPass combined with phacoemulsification had an even more impressive decrease in medication use.6-8
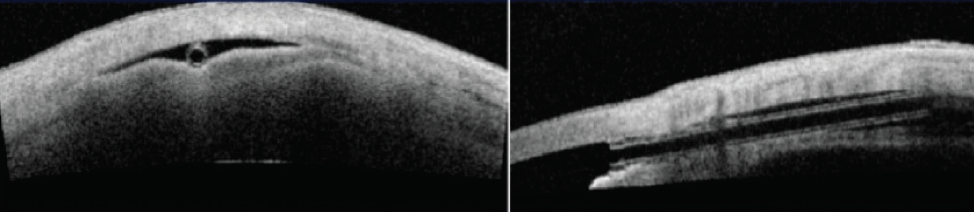
Figure 2. Optical coherence tomography of the CyPass in position with a shallow reservoir./p>
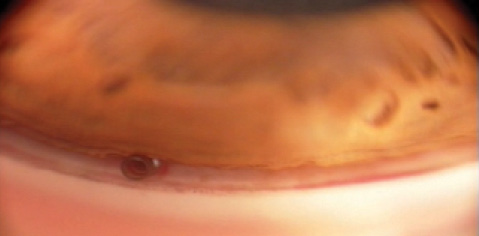
Figure 3. Gonioscopic view of the device.
The pivotal 2-year multicenter US trial enrolled 505 patients, randomized 3:1 for phacoemulsification plus the CyPass compared to phacoemulsification alone. The study included medication-washout IOP results. The trial achieved its primary and secondary endpoints. The device reduced unmedicated IOP by 20% or more in 77% of CyPass microstent subjects versus 60% of controls (P = .001). Mean 24-month medication use was 67% lower in stent subjects (P < .001); 59% of control versus 85% of stent subjects were medication free at 24 months. The device appears to be well tolerated, with no vision-threatening ocular adverse events occurring during the trial; specifically, no cases of endophthalmitis, hypopyon, choroidal detachment or suprachoroidal hemorrhage, or retinal detachment occurred.5 Ultrasound and anterior segment optical coherence tomography imaging show stability of the microstent in the supraciliary space and the presence of localized shallow suprachoroidal reservoirs of aqueous that are not detectable on clinical examination (Figure 2).9 Further investigation into the healing properties and capabilities of the suprachoroidal space itself are of interest now that safe access has been demonstrated (Figure 3).
Given the clinical efficacy and tolerability of the CyPass Micro-Stent, I expect that, when it becomes available in the United States, we will welcome the device as a means of enhancing our ability to customize therapy for our patients.
1. Barkan O, Boyle SF, Maisler S. On the surgery of glaucoma: mode of cyclodialysis. Am J Ophthalmol. 1936;19:21.
2. Toris CB, Pederson JE. Effect of intraocular pressure on uveoscleral outflow following cyclodialysis in the monkey eye. Invest Ophthalmol Vis Sci. 1985;26:1745-1749.
3. Jordan JF, Engels BF, Dinslage S, et al. A novel approach to suprachoroidal drainage for the surgical treatment of intractable glaucoma. J Glaucoma. 2006;15:200-205.
4. Ozdamar A, Aras C, Karacorlu M. Suprachoroidal seton implantation in refractory glaucoma: a novel surgical technique. J Glaucoma. 2003;12:354-359.
5. Vold S, Ahmed II, Craven ER, et al; CyPass Study Group. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts [published online ahead of print August 6, 2016]. Ophthalmology. doi:10.1016/j.ophtha.2016.06.032.
6. Höh H, Grisanti S, Grisanti S, et al. Two-year clinical experience with the CyPass Micro-stent: safety and surgical outcomes of a novel supraciliary micro-stent [in German]. Klin Monbl Augenheilkd. 2014;231:377-381.
7. García-Feijoo J, Rau M, Grisanti S, et al. Supraciliary micro-stent implantation for open-angle glaucoma failing topical therapy: 1-year results of a multicenter study. Am J Ophthalmol. 2015;159:1075-1081.
8. Hoeh H, Vold SD, Ahmed IK, et al. Initial clinical experience with the CyPass Micro-Stent: safety and surgical outcomes of a novel supraciliary microstent. J Glaucoma. 2016;25:106-112.
9. Saheb H, Ianchulev T, Ahmed IK. Optical coherence tomography of the suprachoroid after CyPass Micro-Stent implantation for the treatment of open-angle glaucoma. Br J Ophthalmol. 2014;98:19-23.
Cynthia Mattox, MD
• director, Glaucoma and Cataract Service, New England Eye Center, Boston
• vice chair and associate professor, Department of Ophthalmology, Tufts University School of Medicine, Boston
• (617) 636-8108; cmattox@tuftsmedicalcenter.org
• financial disclosure: investigator in Transcend Medical’s COMPASS US trial; consultant to Alcon and Allergan