
It is fun to play with the latest device and exciting to figure out what can potentially be accomplished with innovative technology. That said, the workbench in my garage is cluttered with tools that are great for a particular purpose but not used often because they are too specialized. I also have a growing collection of gadgets that never lived up to my expectations. There are a few simple tools such as a hammer and screwdriver, however, that I use regularly for their intended purposes but sometimes also for creative, improvised uses.
AT A GLANCE
• Phacoemulsification cataract surgery can be an extremely useful adjunct to the management of IOP in patients with various types of glaucoma. Exactly why IOP drops after cataract surgery, however, remains unclear.
• Depending on the severity of the glaucoma and the target IOP, cataract surgery alone may lower the IOP enough to maintain or improve disease control with a less aggressive medical regimen.
• The role of lens extraction in the treatment of primary angle closure may soon expand.
With all the excitement around the many new devices available for the surgical management of glaucoma, we ophthalmologists cannot overlook the value of the most commonly used basic tool in our anterior segment surgical toolbox. Phacoemulsification cataract surgery has been demonstrated to lower IOP by 1.5 mm Hg (standard deviation ±2.5 mm Hg) after 5 years in healthy eyes without glaucoma.1 Although this decrease may not be appreciated in every eye that undergoes cataract surgery, it can be an extremely useful adjunct to our management of IOP in the glaucoma patient.
WHY DOES IT WORK?
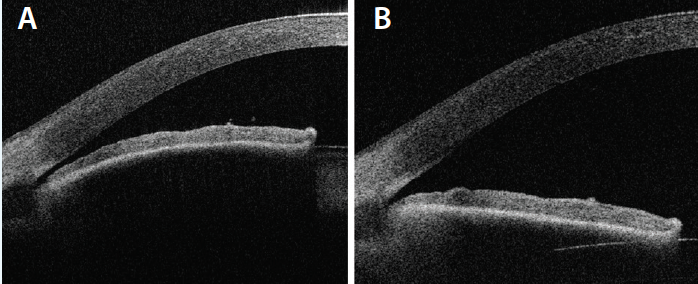
Figure. Anterior segment optical coherence tomography of a PAC eye before treatment (A) and after lens extraction (B).
It is not clear exactly why the IOP drops after cataract surgery. Replacement of the relatively thicker crystalline lens with a slimmer IOL deepens the anterior chamber and widens the angle (Figure).2,3 As the scleral spur relaxes, the trabecular beams may be mechanically pulled open to allow improved aqueous outflow.4 For patients on the primary angle-closure (PAC) spectrum of disease, relief of relative pupillary block contributes to IOP lowering.5 The IOP also decreases in eyes that do not have narrow angles, however, possibly due to trabecular endothelial remodeling by ultrasonic energy from the phaco handpiece6 or mechanical stretching caused by high intraoperative IOP.7
HOW WELL DOES IT WORK?
A recent Ophthalmic Technology Assessment published in Ophthalmology reviewed the effect of phacoemulsification cataract surgery on IOP in patients with primary open-angle glaucoma (POAG), pseudoexfoliation glaucoma, and primary angle-closure glaucoma (PACG). A meta-analysis of nine studies of 461 POAG patients revealed a 13% reduction of IOP and a 12% drop in the number of glaucoma medications. Pseudoexfoliation glaucoma analysis (5 studies, 132 patients) found a 20% decrease in IOP and a 35% reduction in glaucoma medications. The results for PACG (12 studies, 495 patients) were even better: a 30% decrease in IOP and a 58% reduction in glaucoma medications.8
HOW DO I MAKE IT WORK FOR ME?
POAG With Medically Controlled IOP
When a patient with POAG develops a visually significant cataract and undergoes phacoemulsification cataract surgery, I anticipate a modest postoperative IOP reduction. I instruct my cataract patients not to remove their eyeshields until they return to the office the next morning. Because topical glaucoma drops were not used between surgery and the first postoperative visit, I can assess the unmedicated IOP and make decisions based on disease severity and the individualized target IOP. With the infrequent exception of eyes that have elevated IOP on the first day after surgery from retained viscoelastic, I have found that the majority of patients with early glaucoma or ocular hypertension but no glaucomatous damage who were treated with monotherapy can be safely observed for the first week after surgery without needing glaucoma medication. When the patient returns, the IOP has had more time to stabilize, and the treatment plan may need adjustment. Because glaucoma patients are at increased risk of developing steroid responses,9,10 we ophthalmologists must beware of IOP elevation from the routine postoperative regimen. Once the steroid is discontinued, however, an additional IOP reduction may occur. The IOP may eventually return to the higher presurgical levels, but it can be many weeks or months before that happens.
POAG With Borderline IOP
If the severity of glaucomatous damage is more advanced and the IOP is borderline despite treatment with numerous IOP-lowering medications, cataract surgery alone is unlikely to allow discontinuation of all glaucoma eye drops. A decrease in IOP might be enough to stabilize the disease while the same medicinal regimen continues, but if the IOP rises postoperatively, a return to the OR for glaucoma surgery may be necessary. In this group of patients, combining phacoemulsification cataract extraction with traditional trabeculectomy or microinvasive glaucoma surgery may be prudent. If the IOP is already too high when the decision is made to remove a visually significant cataract, combined intervention is advisable.
PAC Spectrum of Disease
If the patient is a PAC suspect or has PAC, the traditional first-line therapy used to relieve pupillary block is laser peripheral iridotomy. Because removal of the crystalline lens is also effective for treating PAC, if the patient has a visually significant cataract, it “kills two birds with one stone” to perform cataract surgery alone. Preoperative laser peripheral iridotomy is generally unnecessary, because the pupillary dilation required to perform cataract surgery will not result in an acute angle-closure attack once the patient becomes pseudophakic during the procedure. If the axial length is so short that there is concern about an acute attack of angle closure in the preoperative holding area, intraoperative intracameral dilating preparations can be administered instead of preoperative eye drops.
The results of the Effectiveness in Angle Closure Glaucoma of Lens Extraction (EAGLE) Study were recently presented at the European Glaucoma Society Congress. The investigators reported that the data went one step further in demonstrating the benefit of clear lens extraction in managing PAC.11 Because the majority of patients on the PAC spectrum are hyperopic, the refractive benefits of making them emmetropic via clear lens extraction are a bonus. Caution is still advisable, though, because intraocular surgery is not risk free, and because there are currently no data comparing complication rates for clear lens extraction in PAC versus routine cataract surgery.
If a PAC patient already has enough trabecular dysfunction to have developed glaucomatous optic neuropathy, removing a cataractous lens may prevent additional narrowing of the angle and possibly halt glaucomatous progression. If the IOP is already elevated despite medication at the time of cataract surgery, a combined glaucoma procedure may be more useful, as with the POAG patient who has borderline or elevated IOP.
CONCLUSION
Cataract surgery can be a particularly useful tool for the management of various kinds of glaucoma. Depending on the severity of the glaucoma and the target IOP, cataract surgery alone may lower the IOP enough to maintain or improve disease control with a less aggressive medical regimen. The role of lens extraction in the treatment of PAC may soon expand as well.
1. Shingleton BJ, Pasternack JJ, Hung JW, et al. Three and five year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients. J Glaucoma. 2006;15(6):494-498.
2. Memarzadeh F, Tang M, Li Y, et al. Optical coherence tomography assessment of angle anatomy changes after cataract surgery. Am J Ophthalmol. 2007;144(3):464-465.
3. Shin HC, Subrayan V, Tajunisah I. Changes in anterior chamber depth and intraocular pressure after phacoemulsification in eyes with occludable angles. J Cataract Refract Surg. 2010;36(8):1289-1295.
4. Hamanaka T, Kasahara K, Takemura T. Histopathology of the trabecular meshwork and Schlemm’s canal in primary angle-closure glaucoma. Invest Ophthalmol Vis Sci. 2011;52(12):8849-8861.
5. Wright C, Tawfik MA, Waisbourd M, et al. Primary angle-closure glaucoma: an update. Acta Ophthalmol. 2016;94(3):217-225.
6. Wang N, Chintala SK, Fini ME, et al. Ultrasound activates the TM ELAM-1/IL-1/NF-kappaB response: a potential mechanism for intraocular pressure reduction after phacoemulsification. Invest Ophthalmol Vis Sci. 2003;44(5):1977-1198.
7. Tumminia SJ, Mitton KP, Arora J, et al. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1998;39(8):1361-1371.
8. Chen PP, Lin SC, Junk AK, et al. The effect of phacoemulsification on intraocular pressure in glaucoma patients: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122(7):1294-1307.
9. Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. II. the effect of dexamethasone in the glaucomatous eye. Arch Ophthalmol. 1963;70492-70499.
10. Becker B, Mills DW. Corticosteroids and intraocular pressure. Arch Ophthalmol. 1963;70500-70507.
11. Azuara-Blanco A. The EAGLE trial: implications for the management of angle closure. Presented at: European Glaucoma Society Congress; June 19-22, 2016; Prague, Czech Republic.
Nicholas Bell, MD
• director of the glaucoma service at the Robert Cizik Eye Clinic of the Ruiz Department of Ophthalmology and Visual Science and the A. G. McNeese, Jr clinical professor of ophthalmology at the McGovern Medical School at The University of Texas Health Science Center at Houston
• (713) 559-5200; nbell@cizikeye.org
• financial interest: none acknowledged


