In pivotal phase 3 trials, resolution of vitreomacular adhesion (VMA) was achieved in about one-quarter of patients 28 days after a single 125-μg intravitreal injection of ocriplasmin (Jetrea; ThromboGenics). Anatomic outcomes can be improved in clinical practice by applying predictive features when selecting patients most likely to benefit from this treatment.
AT A GLANCE
• Treatment options for symptomatic VMA, a progressive, sight-threatening disorder that can lead to irreversible retinal damage and vision loss, are watchful waiting, vietrectomy, or pharmacologic vitreolysis.
• Data suggest that stringent patient selection doubled the likelihood of achieving positive outcomes in patients with symptomatic VMA treated with ocriplasmin.
BACKGROUND
Symptomatic VMA is a progressive, sight-threatening disorder that can lead to irreversible retinal damage and vision loss.1 Treatment options include watchful waiting, vitrectomy, or pharmacologic vitreolysis with ocriplasmin.1 Ocriplasmin is a recombinant truncated human plasmin that degrades extracellular proteins to induce vitreous liquefaction and vitreoretinal separation.2,3 The efficacy and safety of a single 125-µg intravitreal injection of ocriplasmin for treatment of symptomatic VMA was demonstrated in pivotal phase 3 randomized, placebo-controlled trials referred to collectively as MIVI-TRUST.4 The percentage of patients with pharmacologic VMA resolution 28 days after injection was significantly greater in the ocriplasmin group (26.5%) than the placebo group (10.1%; P < .001).4
A post hoc analysis of MIVI-TRUST findings identified five baseline features that are predictive of VMA resolution 28 days after ocriplasmin injection: age younger than 65 years, phakic lens status, VMA diameter less than or equal to 1,500 µm, presence of a full-thickness macular hole (FTMH), and absence of an epiretinal membrane (ERM).5
To determine how these previously identified predictive factors may influence symptomatic VMA resolution rates in a clinical setting, we performed a retrospective analysis of medical records of patients who were treated with a single 125-μg intravitreal injection of ocriplasmin and were monitored with spectral-domain optical coherence tomography (SD-OCT) at our center between February 19, 2013, and November 26, 2013. Results from this retrospective case series analysis demonstrated that stringent patient selection doubled the likelihood of achieving positive outcomes in patients with symptomatic VMA treated with ocriplasmin (unpublished data).
RESULTS FROM CASE SERIES ANALYSIS
Medical records of patients with symptomatic VMA who were treated with a single 125-μg intravitreal injection of ocriplasmin and monitored with SD-OCT were selected for retrospective analysis. Patient consent and institutional review board exemption (New England Institutional Review Board) were obtained. Efficacy parameters analyzed included VMA resolution, FTMH closure, and BCVA change from baseline to final follow-up. Visual acuity was measured in Snellen lines and converted to logMAR for analysis. Safety parameters included ellipsoid zone alterations and subretinal fluid accumulation.
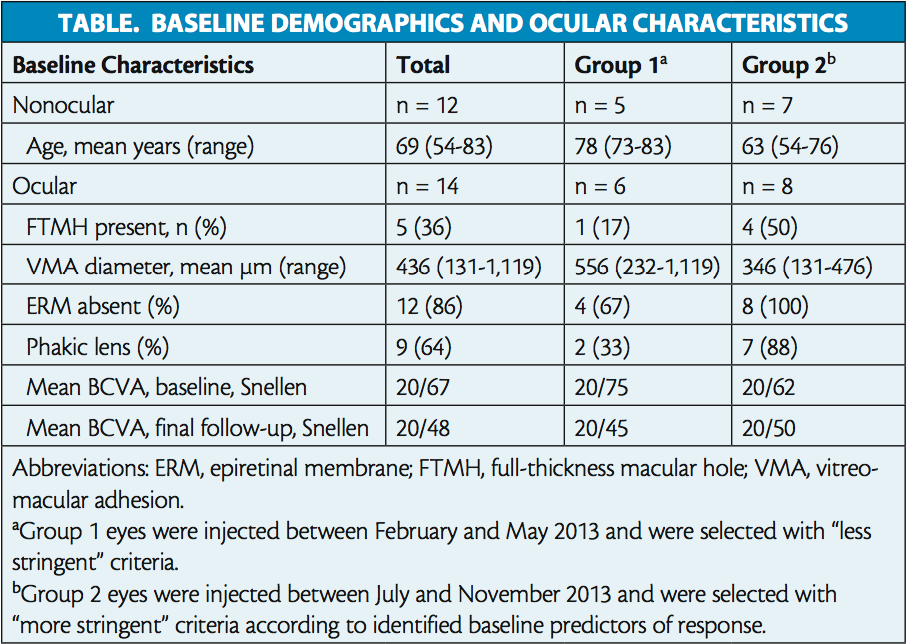
In total, 14 eyes of 12 patients were analyzed. Patient demographics and baseline ocular characteristics are reported in the Table. Of the overall population, half of all eyes (n = 7) achieved VMA resolution, with five cases resolving within 7 days of injection. Among eyes with FTMH at baseline (n = 5), 80% (n = 4) achieved hole closure. Visual acuity gain of more than 2 lines of BCVA was achieved in 29% (n = 4) of all eyes (Figure 1). Mean BCVA changed from 20/67 (0.525 logMAR) at baseline to 20/48 (0.379 logMAR) at final follow-up (Table).
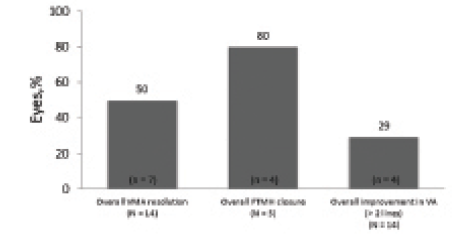
Figure 1. Resolution of VMA, closure of FTMH, and visual acuity change in the entire population.
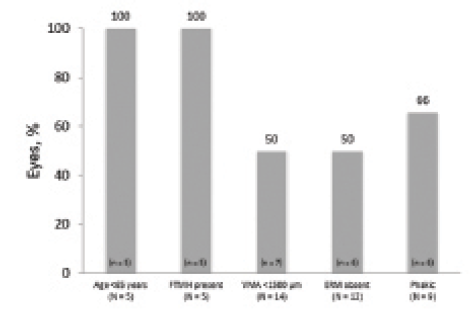
Figure 2. VMA resolution by baseline characteristics.
Resolution rates were then calculated for subgroups defined by the presence of each predictor of response identified in the previous post hoc analysis.5 All eyes of patients who were younger than 65 years (n = 5 of 5) achieved VMA resolution, as did all eyes with baseline FTMH (n = 5 of 5). Resolution was achieved in half (n = 7 of 14) of eyes with focal VMA (< 1,500 μm) and half of those with no ERM at baseline (n = 6 of 12). Two-thirds of phakic eyes (n = 6 of 9) achieved resolution (Figure 2).
To evaluate the effect of more stringent patient selection criteria on symptomatic VMA resolution rates, eyes were divided into two groups based on injection date. Group 1 eyes were injected between February and May 2013 and selected according to “less stringent” criteria. Group 2 eyes were injected between July and November 2013 and were selected according to “more stringent” criteria based on predictors of response (eg, no ERM, FTMH present, and focal adhesion). Retrospective analysis revealed that group 1 eyes were older, were less likely to be phakic or have FTMH, and had larger adhesions (Table). All eyes with ERM (n = 2 of 10) were in group 1. Mean baseline BCVA was 20/75 (0.575 logMAR) in group 1 and 20/62 (0.488 logMAR) in group 2. Mean follow-up time in both groups was 13 weeks. Interestingly, the percentage of patients achieving VMA resolution at final follow-up was nearly doubled in group 2 (63%; n = 5 of 8; Figure 3) compared with group 1 (33%; n = 2 of 6). At final follow-up, mean BCVA was 20/45 (0.350 logMAR) in group 1 and 20/50 (0.400 logMAR) in group 2.
No serious, irreversible adverse events were reported. Most patients reported photopsias and/or floaters, which usually resolved within 1 week. Four of nine eyes had a normal ellipsoid zone at baseline that was altered after injection. More than half of eyes (n = 8) had subretinal fluid after injection. All cases of ellipsoid zone changes and subretinal fluid accumulation fully resolved by final follow-up.
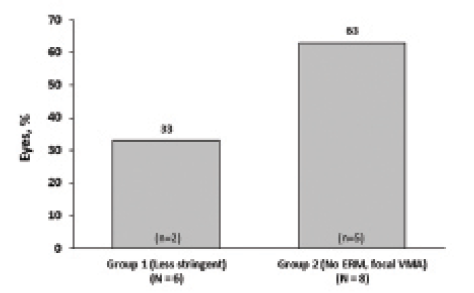
Figure 3. VMA resolution by groups defined by date of treatment and more or less stringent patient selection criteria.
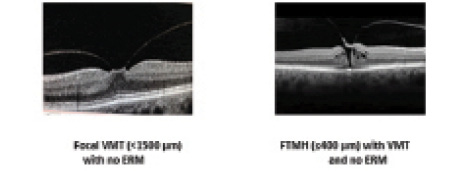
Figure 4. IVTS OCT-based baseline features associated with positive ocriplasmin treatment outcomes.
OCRIPLASMIN PATIENT SELECTION UTILIZING AN OCT-BASED CLASSIFICATION SYSTEM
The baseline features that have been identified as positively associated with higher rates of symptomatic VMA resolution, and shown in this case series to influence patients’ outcomes in a clinical setting, are best observed using OCT imaging (Figure 4). In 2013, a panel of international vitreoretinal experts created an anatomy-based scheme to support consistent definition and classification of VMA-associated pathologies. The International Vitreomacular Traction Study group or IVTS designed a classification system based on OCT anatomic criteria. It excluded clinical features such as fundus findings or symptoms, because they can be subjective in nature.6
According to the IVTS, both VMA and vitreomacular traction (VMT) can be classified as focal (< 1,500 µm) or broad (> 1,500 µm) depending on the diameter of persistent vitreomacular attachment. An FTMH is defined as a foveal lesion that interrupts all layers of the retina from the internal limiting membrane through the retinal pigment epithelium. An FTMH is also classified by the size of the hole (small ≤ 250 µm, medium > 250 to ≤ 400 µm, and large > 400 µm) and the presence or absence of persistent vitreous attachment.6 A standardized classification system such as this allows the consistent identification of appropriate candidates for ocriplasmin treatment and a better understanding of treatment outcomes.
Additional considerations are needed for those patients with symptomatic VMA/VMT associated with an FTMH. The size of the FTMH is an important determinant when selecting patients for ocriplasmin treatment. Data from the pivotal phase 3 trials showed that the success rate was directly proportional to the size of the FTMH; 58% of eyes with an FTMH less than or equal to 250 µm and 37% of eyes with an FTMH between 250 and 400 µm achieved nonsurgical closure. Although investigators intended to exclude eyes with FTMHs larger than 400 µm from the trials, 19 patients with an FTMH larger than 400 µm were treated with ocriplasmin; none of these eyes achieved resolution. It is also important to note that ocriplasmin is not indicated to treat FTMH in the absence of any VMA/VMT and that vitrectomy is the best option for these patients.
CONCLUSION
The critical role of patient selection in optimizing ocriplasmin treatment outcomes is underscored by the identification of baseline predictors of anatomic success. In this retrospective analysis, the clinical population was divided into two groups based on the date of injection, which is a surrogate for the application of “less stringent” or “more stringent” patient selection according to baseline predictors. Rates of VMA resolution after injection were higher among patients who were more stringently selected compared with those who were less stringently selected. Our results from a small case series support the use of predictive features in order to select patients who are most likely to benefit from ocriplasmin injection, and these findings are corroborated by other clinical case series reports.7,8
Meridius Health Communications provided support in manuscript writing and preparation.
Dr. Khanani had full access to all of the case data and takes full responsibility for the integrity of the data and reported findings.
1. Jackson TL, Nicod E, Simpson A, et al. Symptomatic vitreomacular adhesion. Retina. 2013;33(8):1503-1511.
2. Gandorfer A, Rohleder M, Sethi C, et al. Posterior vitreous detachment induced by microplasmin. Invest Ophthalmol Vis Sci. 2004;45(2):641-647.
3. De Smet MD, Valmaggia C, Zarranz-Ventura J, Willekens B. Microplasmin: ex vivo characterization of its activity in porcine vitreous. Invest Ophthalmol Vis Sci. 2009;50(2):814-819.
4. Stalmans P, Benz MS, Gandorfer A, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012;367(7):606-615.
5. Haller JA, Stalmans P, Benz MS, et al; MIVI-TRUST Study Group. Efficacy of intravitreal ocriplasmin for treatment of vitreomacular adhesion: subgroup analyses from two randomized trials. Ophthalmology. 2015;122(1):117-122.
6. Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group. Classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611-2619.
7. Kim BT, Schwartz SG, Smiddy WE, et al. Initial outcomes following intravitreal ocriplasmin for treatment of symptomatic vitreomacular adhesion. Ophthalmic Surg Lasers Imaging Retina. 2013;44(4):334-343.
8. Singh RP, Li A, Bedi R, et al. Anatomical and visual outcomes following ocriplasmin treatment for symptomatic vitreomacular traction syndrome. Br J Ophthalmology. 2014;98(3):356-360.
Arshad M. Khanani, MD
• clinical assistant professor at the University of Nevada School of Medicine and managing partner at Sierra Eye Associates in Reno, Nevada
• arshad.khanani@gmail.com
• financial disclosure: consultant to and speaker for ThromboGenics