Case Presentation
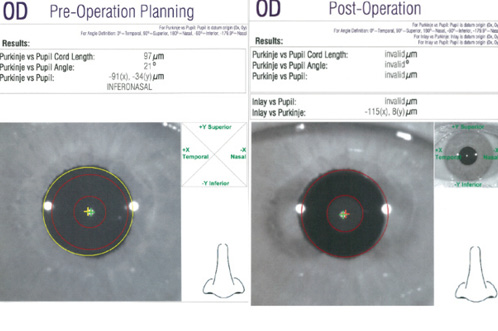
Figure 1. The AcuTarget HD preoperative planning view demonstrates a Purkinje image (green X) versus the center of the pupil (yellow X) and thus the amount of angle kappa. In this case, angle kappa cord length measures 97 μm (A). Four months after surgery, the AcuTarget HD assessment shows the center of the Kamra inlay (red X) in comparison to the Purkinje image (green X). Ideally, the difference between these points is less than 300 μm. In this case, it measures 115 μm nasal and 8 μm superior (B).
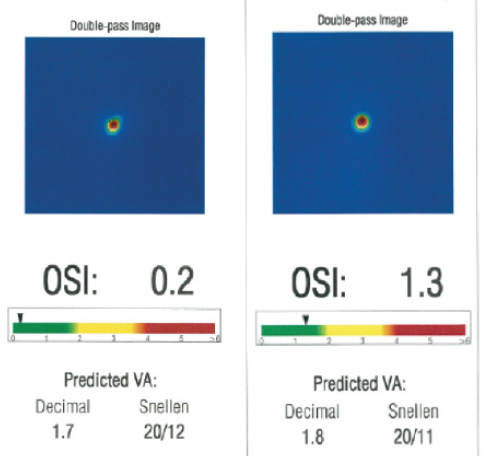
Figure 2. The preoperative OSI measured by the double-pass retina image analysis of the AcuTarget HD was 0.2 (A). Four months postoperatively, the OSI had increased to 1.3 (B).
—Case prepared by William F. Wiley, MD.
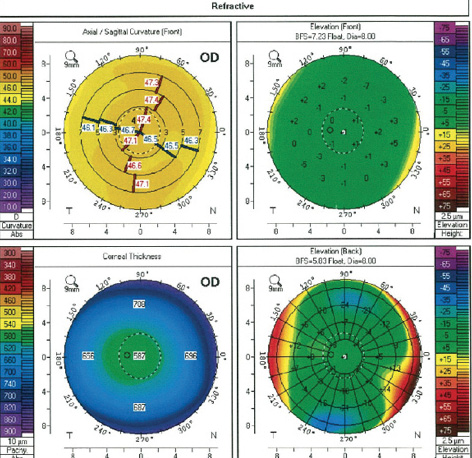
Figure 3. Preoperative topography with the Pentacam showed normal corneal shape and thickness.
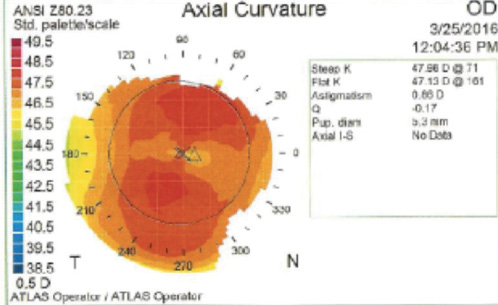
Figure 4. Four months postoperatively, the topographic shape is normal, and there is no apparent central elevation or “red ring” that can be indicative of aggressive corneal wound healing with an accompanying hyperopic shift.

LANCE KUGLER, MD, PCEO
The hyperopic shift of +2.25 D is consistent with an aggressive wound healing response, as is the characteristic “red ring” seen on Placido disc topography, suggestive of peripheral edema. Other symptoms of aggressive wound healing such as photophobia are not present. Nor are slit-lamp examination findings such as corneal haze or edema. The OSI increased from 0.38 preoperatively to 1.3 at 5 months, and pachymetry increased from 582 to 602 µm, suggestive of mild corneal edema that may not be evident on examination. The presence of hyperopia greater than 1.00 D at 3 months suggests that the wound healing response was active at that time and that perhaps restarting steroids would have slowed or reversed the process.
Now at 5 months (2 months after steroid cessation) with a progressive hyperopic shift, mild corneal edema, and no evidence of haze or an infiltrate, it would be reasonable to resume the postoperative steroid regimen while continuing the fixed combination of brimonidine and timolol and to treat the ocular surface aggressively. If under close observation the hyperopia, edema, or topography worsened or did not improve, then I would explant the inlay. If the aggressive wound healing response resolves but the hyperopia persists, then explanation is still warranted, because any further enhancement of refractive error such as hyperopic PRK is best avoided in an eye with this history.

R. LUKE REBENITSCH, MD
This is an interesting case of a usually treatable complication during the postoperative period following implantation of the Kamra. Hyperopic shifts are thought to be the result of keratocyte activation behind the inlay, resulting in a “red ring” on Placido disc topography. They typically occur at least a month after the procedure during the steroid taper, however, rather than within the first few weeks. This patient had a much more robust response to the device, which caused early photophobia and a hyperopic shift.
This patient would have appeared to be an excellent candidate for the Kamra. She had an OSI that was very low at 0.2, exhibited no significant ocular dryness, had lost most accommodative amplitude, and possessed a cornea thick enough for implantation. My initial strategy for management would have been the same as described in the case presentation: increase topical steroids and use serial topographies and midpoint refractions to monitor the response. Unfortunately, this patient was a steroid responder, complicating postoperative management. Given the progressive hyperopic shift and increased IOP, I would recommend explantation of the device and would expect no significant long-term sequelae.
Preventative measures for this ideal candidate are limited. New research, however, suggests that deeper implantation of the Kamra may mitigate some of the risk of a hyperopic shift from the decreased density of keratocytes in the posterior cornea. For a patient with a cornea as thick this patient’s measured with the Pentacam (Oculus Surgical), a pocket of approximately 50% depth may prevent some—or possibly all—of the hyperopic shift she experienced.

GEORGE O. WARING IV, MD
This patient appears to be a great candidate for a Kamra inlay given the refraction and excellent retinal image quality, both statically and dynamically, as determined by double-pass wavefront imaging. These findings are consistent with a diagnosis of stage 1 dysfunctional lens syndrome and a healthy ocular surface.
Her past medical history may give some clues to a nonspecific pro-inflammatory state that we might appreciate retrospectively. Small-aperture inlays tend to perform best with a -0.75 D spherical target, which optimizes near performance without sacrificing distance vision and “cushions” near vision in the event of refractive fluctuation such as is seen here.
Tight spot and line femtosecond laser settings and an adequate low-dose steroid taper over 3 months help to minimize refractive fluctuations; both were used in this case. Careful attention should be paid to IOP for a steroid response, as was observed in this case, resulting in early cessation of the steroid. In most cases, appropriate dosage and duration of a mild steroid will normalize a refractive shift over time.
If a hyperopic shift worsens despite appropriate steroid dosing and duration, thus affecting the patient’s near and distance UCVA and satisfaction, or if the IOP is intractably high for the optic nerve status due to a steroid response, then the inlay should be removed. The removability of the Kamra in the rare event that it is indicated is a true benefit of this “additive” technology. In the US FDA investigational device exemption trial, all eyes that underwent inlay removal retained BCVA.
Section Editor Alan N. Carlson, MD
• professor of ophthalmology and chief, corneal and refractive surgery, Duke University Eye Center, Durham, North Carolina
Section Editor Stephen Coleman, MD
• director of Coleman Vision, Albuquerque, New Mexico
Section Editor Karl G. Stonecipher, MD
• clinical associate professor of ophthalmology, University of North Carolina, Chapel Hill
• director of refractive surgery, TLC in Greensboro, North Carolina
Section Editor William F. Wiley, MD
• private practice at Cleveland Eye Clinic, Cleveland, Ohio
• (440) 526-1974
Lance Kugler, MD, PCEO
• surgeon and CEO of Kugler Vision, Omaha, Nebraska
• director of refractive surgery, University of Nebraska Medical Center
• lkugler@kuglervision.com
• financial interest: none acknowledged
R. Luke Rebenitsch, MD
• refractive surgeon, ClearSight Center, Oklahoma City, Oklahoma
• (405) 733-2020; dr.luke@clearsight.com
• financial disclosure: has received speaking and educational honoraria from AcuFocus
George O. Waring IV, MD
• director of refractive surgery and associate professor of ophthalmology, Storm Eye Institute, Medical University of South Carolina, Charleston, South Carolina
n medical director, Magill Vision Center, Mt. Pleasant, South Carolina
• georgewaring@me.com; Twitter @georgewaring
• financial disclosure: consultant to AcuFocus and Visiometrics


