Glaukos Announces Initial Public Offering
Glaukos announced the launch of its initial public offering of 5,358,000 shares of its common stock. The initial public offering price is currently estimated to be between $13 and $15 per share, according to a company news release. The underwriters for the offering will also have a 30-day option to purchase up to 803,700 additional shares of Glaukos common stock. Glaukos’ common stock has been approved for listing on the New York Stock Exchange under the symbol “GKOS.”
J.P. Morgan, BofA Merrill Lynch, and Goldman Sachs are acting as joint book-running managers for the offering. William Blair and Cantor Fitzgerald are acting as comanagers for the offering.
This offering will be made only by means of a prospectus. A copy of the preliminary prospectus related to the offering may be obtained from J.P. Morgan Securities (e-mail: dg.prospectus_requests@baml.com; or Goldman Sachs, e-mail: prospectus-ny@ny.email.gs.com).
A registration statement relating to these securities has been filed with the US Securities and Exchange Commission but has not yet become effective. These securities may not be sold, and offers to buy may not be accepted prior to the time the registration statement becomes effective. The company’s press release shall not constitute an offer to sell or the solicitation of any offer to buy these securities; nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification of these securities under the securities laws of any such state or jurisdiction.
InSite Vision Completes NDA Submission to the FDA for BromSite
InSite Vision announced that it has completed the submission for a new drug application (NDA) to the FDA for BromSite (0.075% bromfenac) for the treatment of inflammation and prevention of pain in cataract surgery, according to a company news release. InSite is seeking marketing approval of BromSite in the United States. In its confirmatory phase 3 clinical trials, BromSite achieved statistically significant superiority to the vehicle in alleviating ocular inflammation and preventing pain. In such trials, the agent was well tolerated with no significant safety concerns or drug-related serious adverse events reported, according to the company.
The filing of the BromSite NDA satisfies InSite’s obligation under its recently announced merger agreement with Canadian biotech firm QLT. The merger transaction, which has been unanimously approved by the boards of both companies, is subject to the approval of InSite Vision shareholders, a condition that the FDA has not refused to accept the BromSite NDA for review within 60 days after InSite Vision’s filing of the NDA, a condition that the FDA has not indicated that it will require InSite Vision to conduct additional clinical studies prior to the approval of BromSite within 74 days after InSite Vision’s filing of the NDA, and other customary closing conditions. QLT will not require a shareholder vote to conclude the transaction; InSite Vision will file a proxy statement with full disclosure of the transaction and will schedule a shareholder vote to approve the transaction. QLT will provide InSite Vision with a line of credit until the transaction closes. The transaction is expected to close in the third quarter of 2015 and to be taxable to InSite Vision shareholders. Shares of the new company will trade on NASDAQ under the ticker “QLTI” and on the TSX under the ticker “QLT.” To learn more about the merger, visit www.eyewiretoday.com.
Zeiss Presents Its New Generation of Vascular Imaging: OCT Angiography
Carl Zeiss Meditec announced it is developing optical coherence tomography (OCT) angiography for both spectral-domain (SD) and swept-source platforms. The OCT angiography application on the SD platform is currently under FDA review and is 510(k) pending, according to a company news release.
“The en face angiograms from the Zeiss SD-OCT angiography prototype show me a detailed picture of the retinal vasculature of the macula,” Professor Jean-François Korobelnik, chief of the Ophthalmology Service at the Bordeaux Central University Hospital in Bordeaux, France, who has been conducting investigative research on OCT angiography in collaboration with Zeiss, said in the news release. “Being able to visualize so quickly areas of macular ischemia in my diabetic retinopathy patients, without the use of a contrast agent, could really help me more easily determine which diabetic macular edema patients are eligible for treatment.”
According to the company, the progression of eye disease in the posterior segment is often accompanied by changes in the retinal vasculature. Fluorescein angiography (FA), the gold-standard imaging modality for visualizing such changes, has two main limitations: it requires the use of an injected contrast agent, and it captures the entire retinal vascular volume without the ability to segment imaged features by their depth.
Zeiss said that OCT angiography aims to address these limitations by enabling easy, high-resolution depth-resolved visualization of the separate layers of the retinal and choroidal vasculature. Without the need for a contrast agent, OCT angiography reportedly offers physicians a quick and relatively low-risk alternative to FA to clearly visualize blood flow. OCT angiography detects the motion of scattering particles such as red blood cells within sequential OCT B-scans performed repeatedly at the same location of the retina. Zeiss is able to utilize its retinal tracking system, FastTrac, to provide motion-artifact-free images of the perfused retina.
A study by Zeiss and its research collaborators directly compared OCT angiography (using a prototype based on the Cirrus HD-OCT 5000) with FA imaging, in a subject who had diabetic retinopathy.1 There was a clear correlation between the OCT angiography and fluorescein images, plus OCT angiography was found to reveal much clearer microvascular details, according to Zeiss. OCT angiography also provided depth-resolved information that was not available with FA.
The research featured collaboration with researchers from leading institutions, including the University of California, Los Angeles, the University of Miami Health System, the University of Southern California, and the University of Washington.
“It is an exciting time in ophthalmology with the advent of OCT angiography,” Giovanni Staurenghi, MD, professor of ophthalmology and chairman of the University Eye Clinic at Luigi Sacco Hospital in Milan, Italy, who is conducting OCT angiography research in conjunction with Zeiss, said in the news release. “It allows us to quickly and comfortably visualize the vasculature of the retina without introducing the risks associated with a contrast agent.”
- Lin A, Durbin MK, Lee S, et al. Quantitative and qualitative evaluation of diabetic retinopathy retinal vasculature with Cirrus-5000 Angiography prototype. Poster presented at: The Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting; May 5, 2015; Denver, CO.
Caribbean Pearls for Your Practice
The Radial Tenotomy
By Lance S. Ferguson, MD
Surgeons who advocate near-clear vascular incisions tout their superiority in terms of wound stability (surgically induced astigmatism predictability), wound healing, and decreased risk of endophthalmitis. The clear corneal crowd dislikes the attendant subconjunctival hemorrhage and, particularly, the intraoperative chemosis that can confound visualization when irrigation fluid pools in the induced limbal trough. The negative tear film meniscus obscures anterior segment detail and can be a contributing factor in intraoperative complications.
The solution? Our technicians watch the peri-incisional conjunctiva during phacoemulsification, and as soon as any conjunctival ballooning presents, they simply say, “Westcott.” This is my cue to pause, remove my nucleus rotator, and with that hand, use blunt Westcott scissors to create two 3.5-mm incisions through the conjunctiva perpendicular to the globe, radial to the center of the cornea, and immediately adjacent to each side of the incision (Figure). These radial tenotomies will arrest any further progression of chemosis, bleed minimally, and preserve excellent visualization for the remainder of the case. The maneuver is simple, taking only 5 to 7 seconds. The tenotomies are left unsutured, have no effect on surgically induced astigmatism, and are practically invisible at the slit lamp on postoperative day 1.
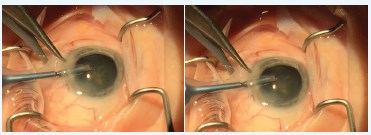
Figure. Radial incision with Westcott scissors
If the incisions are created after significant chemosis has occurred, they will be less effective. I find it best to incise early after presentation.
Snip, snip, and see.
To learn more about and to register for the 2016 ACES/SEE Caribbean Eye Meeting, visit www.caribbeaneyemeeting.com.
Lance S. Ferguson, MD
• Commonwealth Eye Surgery, Lexington, Kentucky
• lferguson@commonwealtheyes.com
ONL Therapeutics’ Photoreceptor Protection for Retinal Diseases
ONL Therapeutics provided an update on the company’s ongoing drug development program to block apoptosis, which occurs in a range of retinal diseases and is the root cause of vision loss and the leading cause of blindness. The company’s recent research and development efforts have led to a number of milestones, including the promotion of a new lead development candidate and the expansion of its planned clinical program to include both dry and wet forms of age-related macular degeneration based on promising preclinical in vivo data, according to a company news release.
As a result of ongoing research, ONL recently identified and patented a novel, first-in-class small-molecule peptide, ONL1204, with an attractive profile for inhibiting the Fas pathway, a primary mediator of photoreceptor apoptosis. n